Abstract
Spinal cord injury (SCI) can lead to dramatic physiological changes which can be a factor in developing secondary health conditions and might be reflected in biomarker changes in this elevated risk group. We focused specifically on the endocrine and inflammation profile differences between SCI and able-bodied individuals (ABI). Our aim was to determine the differences in inflammatory markers and endocrine profiles between SCI and ABI. We systematically searched 4 electronic databases for relevant studies. Human observational (cross-sectional, cohort, case–control) studies that compared biomarkers of interest between SCI and ABI population were included. Weighted mean difference between SCI and ABI was calculated using random-effects models. Heterogeneity was computed using I2 statistic and chi-squared test. Study quality was evaluated through the Newcastle–Ottawa Scale. The search strategy yielded a total of 2,603 studies from which 256 articles were selected for full-text assessment. Sixty-two studies were included in the meta-analysis. SCI individuals had higher levels of pro-inflammatory C-reactive protein and IL-6 than ABI. Creatinine and 25-hydroxyvitamin D3 levels were lower in SCI than ABI. Total testosterone levels and IGF-1 were also found to be lower, while cortisol and leptin levels were higher in SCI when compared to ABI. Accordingly, meta-regression, subgroup analysis, and leave-one-out analysis were performed, however, they were only able to partially explain the high levels of heterogeneity. Individuals with SCI show higher levels of inflammatory markers and present significant endocrinological changes when compared to ABI. Moreover, higher incidence of obesity, diabetes, osteoporosis, and hypogonadism in SCI individuals, together with decreased creatinine levels reflect some of the readily measurable aspects of the phenotype changes in the SCI group. These findings need to be considered in anticipating medically related complications and personalizing SCI medical care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinal cord injury (SCI) refers to any traumatic or non-traumatic damage to the spinal cord that leads to motor, sensory, and/or autonomic impairments below the affected area [1]. SCI specifically compromises the central nervous system (CNS), vasculature and blood–spinal cord barrier [2]. Worldwide, the prevalence of SCI is estimated from 236 to 4,187 per million inhabitants, with a growing incidence rate of 133 to 226 thousand cases of traumatic SCI per year [3]. Although the incidence is relatively low compared to other diseases, there is a tremendous economic burden for the healthcare system due to the high prevalence of secondary health conditions and the need for chronic care [4, 5]. Overall, SCI individuals have increased mortality and morbidity rates when compared to age-and sex- matched able-bodied individuals (ABI) [6].
Furthermore, the injury confers significant physiological changes that could lead to the development of various health conditions [7,8,9]. Individuals with SCI have a higher incidence of developing cardiovascular diseases (CVDs), pressure ulcers, neurogenic bladder, recurrent urinary tract, skin, and respiratory infections, as well as, several metabolic disorders [7, 8, 10]. SCI individuals are characterized by a constant low-grade chronic inflammatory state, having a main effect in post-SCI complications [11]. A prolonged inflammatory state leads to the release of proinflammatory cytokines which alter the functionality of barriers, tissues and organs, and consequently exposing the body to unfavorable conditions [12]. Low-grade chronic inflammation therefore, has a significant effect on neurodegeneration and functional recovery after SCI [13]. Moreover, circulating levels of inflammation markers such as C-reactive protein (CRP) and interleukin-6 (IL-6) have been shown to be significantly increased in individuals with chronic SCI, regardless of the duration and level of injury, when compared to ABI [11].
In addition, the loss of neurologic control from the injury leads to muscle atrophy and bone loss. This results in limitations on mobility and functioning of the individual that has ramifications on the development of other chronic diseases. Furthermore, the SCI population undergoes premature aging and accelerated deterioration of body systems that are also manifested by endocrine and inflammatory profile differences when compared to ABI [14,15,16]. SCI individuals not only experience a decline of several anabolic and somatotropic hormones -especially sex hormones- [18], but also have immune dysfunction [19]. Suppressed function of natural killer cells, neutrophils, macrophages and lymphocytes has been evidenced after SCI [20].
Several small studies have been conducted to characterize the inflammatory and endocrine profile of individuals with SCI [21,22,23,24,25,26]. These studies show varying effect estimates due to small study sizes and different comparison groups. In addition, some studies also show no significant differences among SCI and ABI individuals or present inconsistent findings amongst each other. Although there are a number of studies on hormones and inflammatory profiles in individuals with SCI [21,22,23,24,25,26], there have been no efforts to appraise the literature and synthesize the data. Thus, we reviewed the literature to determine the differences in inflammatory markers, hormones, and other related metabolites in individuals with SCI compared to ABI. Furthermore, we discussed the gaps in the literature to guide future research on biomarkers for health screening and promotion in SCI and ultimately provide basis to optimize medical care in this group.
2 Methods
This is a systematic review and meta-analysis of observational studies that reported various inflammatory markers, hormones, and other metabolites levels between SCI and ABI.
2.1 Data sources and search strategy
We conducted the review following a recently published guideline for systematic reviews and meta-analysis [27] and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [28]. The electronic search was performed using the following databases: MEDLINE (Ovid), EMBASE, Cochrane CENTRAL, and PubMed from inception until September 21, 2020 (date of the last search). In addition, the first 100 hits in Google Scholar were included to further the scope for eligible studies. The detailed search strategy was developed by medical information specialists following standard blood panel recommendations [29] and can be found in the Supplementary Information (SI) [30]. Studies comparing the following outcomes between SCI and ABI were included, (a) inflammatory markers (C-reactive protein, CRP; high sensitivity CRP, hsCRP; interleukin-6, IL-6; tumor necrosis factor-alpha, TNF-alpha), (b) insulin, (c) creatinine, (d) vitamin D [hydroxy -25(OH); dihydroxy 1,25 (OH)], (e) fasting glucose and (f) hormones and growth factors (total testosterone; free testosterone; thyroid-stimulating hormone, TSH; triiodothyronine, T3; luteinizing hormone, LH; follicle-stimulating hormone, FSH; growth hormone, GH; cortisol; adrenocorticotropic hormone, ACTH; adiponectin; aldosterone; insulin growth-like factor 1, IGF1; leptin; prolactin; parathyroid hormone, PTH; sex-hormone-binding globulin, SHBG). The full review protocol can be accessed online (International Prospective Register of Systematic Reviews, PROSPERO No. CRD42020210685).
2.2 Study selection, eligibility criteria, and data extraction
We included observational studies (i.e., cross-sectional, cohort, case–control studies) on adults that compared inflammatory and endocrine levels markers between SCI and ABI. We excluded clinical trials, mechanistic studies, experiments on cells (in-vitro models), and animal studies (in-vivo models). Reviews, conference abstracts, cost-effectiveness, economic assessments, letters to editors, commentaries, and other non-peer-reviewed articles were excluded. No date and language restrictions were applied. Titles and abstracts were screened by at least two independent reviewers (GB/PFR and EV/AB/GPF/OAI). Full-text articles were extracted and reviewed for eligibility by two authors (GB and PFR). Adjudication was done by a third author (MG/JS) if consensus between the two reviewers could not be reached. Data extraction was performed separately by the two reviewers using a previously established template.
2.3 Quality of evidence assessment
Study quality and risk of bias were assessed following the Newcastle–Ottawa Scale for cross-sectional, case–control, and cohort studies by the two reviewers (GB and PFR) [31]. In summary, this scale is based on three categories: (a) study selection (i.e., sample size, representativeness of the sample, and ascertainment of exposure), (b) comparability (i.e., factors that were compared between the groups other than the outcome), and (c) outcome (assessment and the statistical test used). A rating was given per category for each study with a maximum total score of 10. Studies with scores of 8–10 were classified as high quality, 5–7 as moderate quality, and 1–4 as low quality [27].
2.4 Data synthesis and analysis
Biomarkers-of-interest levels, including mean and standard deviation (or standard error of the mean), median and interquartile range (or minimum and maximum values) were extracted. For data reported as medians, ranges, or 95% confidence interval; the means and standard deviations were calculated using previously defined methods [32]. Only baseline values were used in studies with repeated measures. For outcomes that were reported in different units, we converted the values into the International System of Units (SI) [33]. We computed for pooled means and standard deviation of SCI and ABI groups. Weighted mean difference (WMD) between SCI and ABI biomarker values was computed using the random-effects model by DerSimonian and Laird method [34]. A positive WMD signified that the pooled mean of individuals with SCI is higher than in ABI. A negative value meant that individuals with SCI have lower pooled means compared to ABI.
Study characteristics (i.e., location of the study, number of participants, source of control population, matching variables), participant characteristics (i.e., age, sex, health status), and injury characteristics (i.e., duration of injury, injury completeness, injury level) were collected from each study. Heterogeneity was evaluated using the Cochran's squared test (x2) and Higgins I2 statistic test. The level of heterogeneity was classified as low (I2 ≤ 25%), moderate (I2 > 25%, < 75%), or high (I2 ≥ 75%) [35]. To further explore heterogeneity, we did stratified analyses using study, participant, and injury characteristics as strata. We also used random-effects meta-regression for study, participant, and injury characteristics with continuous data. Both stratified analysis and meta-regression were conducted for outcomes with ten or more studies. A leave-one-out analysis was also performed to evaluate if a single study affects the overall weighted mean difference. This analysis iteratively removes one study at a time and recomputes the weighted mean difference to detect any notable variation. Publication bias was explored through funnel plots and Egger's test for outcomes with more than eight studies [36]. STATA 16.1 (Stata Corporation, College Station, Texas) was used for statistical analysis and p-values < 0.05 were considered statistically significant.
3 Results
3.1 Literature search and study characteristics
The search strategy yielded 4,464 records, from which 1,861 were removed as duplicates. We screened 2,603 titles and abstracts, from which 256 articles were selected for full-text assessment, Fig. 1. Three additional citations were manually searched and included in the pool of included studies. Overall, 62 studies provided information on targeted outcomes in both ABI and SCI individuals and were included in the meta-analysis. A detailed summary of the studies included in the meta-analysis can be found in Table S1 [30], and the reason for the exclusion of other studies can be found in Table S2 [30].
Study characteristics of all studies included in the meta-analysis can be found in Table 1. Most of the studies were conducted among males (42/62, 68%), had a sample size below 100 participants (46/62, 74%), included both individuals with tetraplegia and paraplegia (48/62, 77%), and were conducted in North America (25/62, 40%). The mean age ranged from 16 to 64 years and the mean injury duration ranged from < 1 to 29 years. One-third of the studies (19/62, 31%) included complete injury, 40% (25/62) studies had both complete and incomplete injury, while 29% (18/62) of studies had no information. Most of the studies were classified by the Newcastle–Ottawa scale as moderate quality (44/62, 71%), while the rest of the studies were classified as good quality (18/62, 29%) Table 1.
3.2 Inflammatory markers
Based on the findings from 13 studies on inflammation-related markers including 532 SCI and 437 ABI individuals, we found that IL-6 (WMD 2.52 pg/mL, 95% confidence interval (CI) 1.82, 3.21), I2 81.1%, pχ2 0.001) and CRP (WMD 2.79 mg/L, 95% CI 1.75, 3.83, I2 87.3%, pχ2 < 0.001) were significantly higher in the SCI group than the ABI group. No significant differences were found between the two groups regarding hsCRP and TNF-α.
3.3 Creatinine and vitamin D
From the seven studies comprising 260 SCI and 136 ABI, creatinine was found to be significantly lower (WMD -14.23 µmol/L, 95% CI -21.57, -6.89, I2 90.3%, pχ2 < 0.001) in the SCI group compared to ABI. Five studies with 289 SCI and 123 ABI individuals compared vitamin D profiles between SCI and ABI populations. The 25-hydroxyvitamin D3 [25(OH)D] was significantly lower (WMD -10.32 nmol/L, 95%CI -20.47, -0.18, I2 57.2%, pχ2 0.053) in SCI individuals compared to ABI. No significant difference was found in 1,25-dihydroxyvitamin D [1, 25(OH)D] levels.
3.4 Insulin and glucose
Twenty-two studies compared glucose and insulin profiles (1,073 SCI and 1,003 ABI), however, no significant differences between the two groups were found either in glucose (WMD -0.08 mmol/L, 95% CI -0.19, 0.03, I2 83.8%, pχ2 < 0.001) or insulin (WMD 3.99 pmol/L, CI -2.84, 10.83, I2 50.5%, pχ2 0.019).
3.5 Hormones and growth factors
Thirty-seven studies explored hormone and growth factors profiles between 1,149 SCI and 918 ABI. Results from the meta-analysis showed significantly lower total testosterone (WMD -2.61 nmol/L, 95% CI -4.42, -0.79, I2 89.6%, pχ2 < 0.001) for the SCI group than ABI, however free testosterone (WMD -0.01 nmol/L, 95%CI -0.024, 0.004, I2 81.8%, p < 0.001) was not found to be significantly different. Decreased levels were also found for IGF-1 (WMD -6.82 nmol/L, 95% CI -9.24,-4.40, I2 0%, pχ2 0.529). On the other hand, cortisol levels (WMD 103.43 nmol/L, 95%CI 10.75, 196.11, I2 67.5%, pχ2 0.026) and leptin levels (WMD 0.19 nmol/L, 95%CI 0.10, 0.27, I2 53.1%, pχ2 0.047) were shown to be higher in SCI individuals when compared to ABI. No significant differences were found in TSH, T3, LH, FSH, GH, ACTH, adiponectin, aldosterone, prolactin, PTH, and SHBG.
3.6 Heterogeneity analysis, meta-regression, subgroup analysis and sensitivity analysis
Significant study heterogeneity (I2 > 75% and Cochran x2 p < 0.05) was found in weighted mean differences for CRP, IL-6, TNF-α, creatinine, 1, 25(OH)D, total testosterone, T3, LH, FSH, prolactin, PTH and SHBG (Table 2). We further performed subgroup analysis using study design, injury, and participant characteristics as strata (Table S3−S7) [30]. Time since injury and age-sex matching in the meta-analysis of FSH (Table S5) [30] and location and health status in the meta-analysis of insulin (Table S6) [30] were identified as potential sources of heterogeneity. We were not able to explain other sources of heterogeneity in cases where I2 was higher than 75% (Tables S3–S9) [30]. In meta-regression, number of participants was identified as another potential source of high heterogeneity in total testosterone, LH, and FSH. Larger studies tended to have lower weighted mean difference (β -0.040, 95% CI -0.075, -0.005), FSH (β -0.064, 95% CI-0.100,-0.027), and LH (β-0.064, 95% CI -0.100, -0.027) in SCI compared to ABI (Table S9 and Fig. S3) (17). Leave-one-out sensitivity analysis showed that our effect estimates were stable upon iteratively removing one study at a time. No single study influenced our overall estimates (Fig. S2) [30].
3.7 Study quality and publication bias
Publication bias was explored for total testosterone, LH, FSH, CRP, and insulin Fig. S1 [30]. All funnel plots were qualitatively symmetrical suggesting no publication bias. Similarly, Egger's tests on testosterone, LH, FSH, CRP, and insulin had p > 0.05, thus we were less likely to miss smaller studies (Fig. S1) [30].
4 Discussion
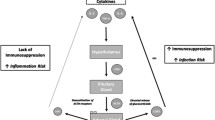
Determining the differences in inflammatory markers and endocrinological profiles of individuals with SCI from ABI could lead to a better understanding of the physiologic changes after SCI and could aid in anticipating complications, and prevention of secondary health outcomes, and rehospitalizations for this high-risk group. We showed that individuals with SCI have higher inflammatory marker levels (CRP and IL-6) compared to ABI. Moreover, we also observed lower creatinine and vitamin D in SCI compared to ABI, which points to changes in body composition. For hormones, total testosterone and IGF-1 were lower in individuals with SCI compared to ABI, while cortisol and leptin were found to be higher. Thus, individuals with SCI showed evidences of chronic inflammation, accelerated muscle and bone loss, and endocrinological alterations of anabolic and catabolic hormones when compared to ABI (Table 3 and Fig. 2).
Cytokines are proteins that originate from immune cells, specifically macrophages, and monocytes, at the place of inflammation [37]. We found higher levels of pro-inflammatory cytokines in SCI compared to ABI, particularly CRP and IL-6, which is consistent with the findings from other studies [38, 39]. CRP is known to be an acute phase reactant found at higher levels during the early stages of injury, infection, or other inflammatory stimuli [11]. IL-6 is another cytokine involved in the innate and adaptive immune responses which mediates CRP production [11]. Thus, we find that the injury leads to prolonged higher baseline inflammation levels. There may be at least three plausible explanations for this findings. First, inflammation is the expected physiological response to recurrent urinary, skin, and respiratory infections that are common among individuals with SCI [10]. Second, individuals with SCI are predisposed to higher fat accumulation. Obesity and overweight were observed at rates of 29.9% and 65.8% in SCI and were higher compared to ABI resulting in an increased accumulated volume of adipose tissue [40, 41]. Higher adiposity leads to the release of proinflammatory mediators such as adipokines IL-6 and TNF-α. [42]. Third, there is a close relationship between inflammation and the endocrinologic profile in the SCI group, particularly regarding glucocorticoids and corticotropins [13, 20]. Proinflammatory cytokines can upregulate the hypothalamus–pituitary–adrenal (HPA) axis [13, 20]. A chronic imbalance within the HPA axis in conjunction with hormonal dysregulation may result in immune dysfunction. In an overactivated state of the HPA axis, the corticotropin hormone from the hypothalamus could lead to the release of ACTH from the pituitary gland, followed by the release of glucocorticoids such as cortisol [20]. Overproduction of glucocorticoids may provoke immunosuppression [20]. Such mechanisms could explain the elevated levels of cortisol in individuals with SCI found in our results and other studies [43,44,45]. Most of the studies, show increased cortisol levels in acute stage of the injury, however, our findings showed also chronically elevated cortisol levels, possibly due to the chronic low-grade inflammatory state in SCI individuals [20]. Furthermore, the adrenal gland which is under the control of the sympathetic trunk from the thoracolumbar spine could be damaged after SCI injury and may affect the balance of steroids hormones [20].
Differences between SCI and ABI individuals regarding sex steroids were also identified in our study. We found lower testosterone levels in individuals with SCI. The literature suggests that the sympathetic innervation of lymphoid organs could also be affected after injury [20]. Low levels of testosterone were observed alongside a high prolactin level [46] and low LH [47], which may suggest a dysfunction of the HPA. In our analysis, two studies observed lower LH in individuals with SCI, but pooled estimates of 15 studies did not reveal any statistically significant difference. Aside from the injury, low levels of testosterone were also associated with several coexisting factors, including age, medication use, obesity, and other clinical comorbidities [21, 48]. Furthermore, hypogonadism has also been reported to be more prevalent amongst SCI individuals compared to age-matched ABI [49].
Moreover, individuals with SCI have altered body composition, specifically lower muscle mass and higher adiposity, when compared to the general population. These conditions are compatible with sarcopenic obesity. Sarcopenic obesity is commonly seen in the elderly and in cancer patients [50, 51] and has been described in the SCI population [52]. In SCI, etiology for sarcopenic obesity is multifactorial and it is characterized by increased leptin levels, low vitamin D, and lower muscle mass, using creatine as a surrogate measure [53]. Creatinine as a metabolite of muscle breakdown is respectively decreased in chronic SCI individuals who have reduced muscle mass due to the increased muscle loss and atrophy from the denervation and reduced physical activity after the injury [54, 55]. Though, SCI individuals experience not only muscle loss but also bone loss. Vitamin D is not only essential for calcium absorption and metabolism but also for bone mineralization which is directly associated with bone mineral density (BMD) [56,57,58,59,60,61]. SCI individuals show lower vitamin D levels compared to the general population. Moreover, the lack of weight-bearing, diminished limb use, and chronic inflammation associated with the injury also contributes to higher bone loss and bone turnover. Several studies have established these osteopenic changes and this is considered as one of the major health conditions needing attention in this group [62, 63]. Additionally, Vitamin D deficiency has also been linked to other pathological conditions, including autoimmune and inflammatory diseases [64]. Vitamin D, is responsible for decreasing the production of type 1 proinflammatory cytokines and enhancing the generation and activation of type 2 anti-inflammatory cytokines, as well as, T-regulatory cells and tolerogenic dendritic cells [64].
Furthermore, we found lower IGF-1 levels in SCI population compared to the general population, similar to other studies [65, 66]. IGF-1 promotes normal bone and muscle growth [18]. Decreased levels of IGF-1 have been related to the presence of skeletal muscle atrophy and higher fat mass accumulation [8]. A decline in IGF-1 may be also indicative of the development of sarcopenia, which is characterized by a progressive and general loss of skeletal muscle mass and strength [18]. Moreover, we also found higher leptin levels in SCI individuals, similar to other studies [8, 67, 68]. Leptin is released by adipose tissue and past studies have confirmed higher fat accumulation in SCI individuals explaining the higher leptin levels in this group [8]. Leptin levels are 32% higher in persons with SCI compared to ABI [8].
Due to body composition changes and an associated poorer lifestyle after the injury individuals with SCI have also a younger onset and higher incidence of diabetes compared to the general population [69,70,71]. Furthermore, testosterone deficiency has been associated with higher diabetes predisposition in men [72, 73]. Nonetheless, our results did not show any significant differences in glucose and insulin between these two populations. This can be potentially explained based on three grounds. First, the majority of the studies reported only fasting glucose and insulin levels. Second, in most of the studies in which these outcomes where measured insulin resistant or glucose intolerant individuals were excluded. Lastly, further analysis could not be done as diet, exercise, and antidiabetic medication were not reported—a possible explanation for the high heterogeneity of our pooled estimates. A potential solution would be the use of fasting glucose in combination with 2-h post load glucose (2 h-PG) and hemoglobin A1c (HbA1c) as diagnostic measures, which has proven to be more efficient in predicting the risk of incidence of diabetes and should be considered in future studies [74].
Overall, there are significant differences in inflammatory and endocrine profiles in individuals with SCI compared to the general population. These differences can be directly associated with SCI or derived from secondary health conditions developed post-injury. Alternatively, they could also be viewed as the product of a normal compensatory process as these individuals transition into a new equilibrium. In the initial phases of SCI, compensation could be characterized by intrinsic functional compensation. However, over time, structural changes may occur in order to achieve metabolic homeostasis [75]. As an example, we can expect lower creatinine levels in the SCI population because of reduced muscle mass as a consequence of decreased levels of physical activity and muscle denervation. Nonetheless, decreased levels of anabolic hormones after SCI could also be responsible for the deterioration in body composition or other related metabolic profile disorders and lead to a decreased capacity of cellular repair and maintenance of lean muscle mass and strength [8]. Moreover, since SCI individuals tend to age prematurely, we anticipate a decline of several anabolic and somatotropic hormones, especially sex hormones [17, 18].
Our review synthesized inflammatory and endocrine markers to characterize the physiologic profile in SCI. To the extent of our knowledge, this systematic review and meta-analysis is the first in the literature that focused on inflammatory markers, hormones, and other related metabolites in SCI. Most of the reviews have been focused on specific biomarkers, no systematic literature search, or have had no quantitative synthesis. We used state-of-the-art search syntax and used meta-analysis tools to compute for weighted mean difference and investigate heterogeneity. We collected information on biomarkers from studies which in their majority contained age-and-sex matched individuals. Furthermore, most research in SCI has been based on small sample size resulting in inconsistent effect estimates across studies. We pooled results from different studies to increase the statistical power and precision of the effect estimates. Finally, we provided an absolute value on the difference that could be useful in future research.
However, our analysis had several limitations that need to be considered while interpreting the results. First, our analysis was based on cross-sectional studies. These studies are snapshots of a particular point in time and the directionality of the exposure (injury) and outcome (endocrine and inflammation markers) are difficult to establish. Second, we pooled results from different studies with varying primary outcomes. This may have contributed to high heterogeneity in our findings. We performed meta-regression, subgroup analysis, and leave-one-out, albeit still insufficient in explaining the high heterogeneity. Third, the pooled estimates were not adjusted for lifestyle factors which include physical activity, smoking, medication use, and nutritional intake. Studies did not provide standardized reporting of such lifestyle factors precluding further exploration. Fourth, results were also mostly based on SCI individuals with chronic injury. We cannot speculate at what time point after the injury the physiological changes occur. Finally, our findings are based mostly on males and North American cohorts, which may not be generalizable to other populations.
Future studies should focus on other biomarkers that elucidate the understanding of the physiological changes that SCI carries and optimize prognosis and diagnosis of specific health conditions that derive from the primary injury. For instance, given the fact that the SCI population has a higher risk of developing CVD's, it is important to mention that even though hsCRP, which is indicated specifically for cardiovascular risk assessment, because of its superior assay precision and accuracy, it is nonetheless much less studied than CRP, or less encountered due to bias by indication [42]. Another example is the use of cystatin-c simultaneously with creatinine to estimate renal function in SCI individual, since cystatin-c is not a marker correlated to muscle mass [76]. Furthermore, biomarkers are dynamic and capture a health status at a specific point in time. Therefore, more longitudinal studies are needed to reflect changes in biomarkers over time. In addition, given the individual objectives and research focus of most studies, these were only conducted in males, leading to a lack of female-specific and female-specific risk factors studies. Sex plays a major role in the differences between inflammatory markers and hormones in ABI. For example, few studies have ventured into estradiol and estrogen-derivative hormone levels in SCI and its neuroprotective effects. Estrogen has been shown to increase revascularization, reduce inflammation, reduce oxidative damage, and downregulate the apoptotic pathways in some neurologic conditions [77,78,79]. A few studies have also shown neuroprotective effects of estrogen supplementation in SCI animal models, although the translation of it into clinical use is yet to be explored. Because few women are involved in most of the studies, clinical trials on the use of estrogen is made more challenging. The underrepresentation of females in SCI conducted studies further emphasizes the need to adopt more sex- and gender-sensitive research frameworks to explore determinant of health in females [80]. Finally, post-injury rehabilitation aims to train gross and fine motor function, and further increase independence in activities of the daily living [81]. Future studies should explore the association between rehabilitation aspects (functional recovery, mobility) and endocrinological and inflammatory profiles.
5 Conclusions
SCI results in dramatic physiological changes as a direct result of the injury or by consequence of secondary condition impairments caused by the injury. Our systematic review has shown that individuals with SCI have a higher level of inflammation and significant endocrinological changes reflected not only in higher muscle and bone loss but also in the presence of other metabolic diseases. These findings are aspects of the physiological profile of the SCI population, which need to be considered in anticipating the medical problems and optimizing medical care in this group. As metabolic and endocrine function is altered in SCI individuals, regular screening on diabetes, osteoporosis and hypogonadism is recommended to always be included in patient management guidelines [82]. Moreover, we know that SCI individuals undergo a compensatory process in order to reach metabolic homeostasis, these changes however, do not necessarily indicate the presence of a pathology and could be considered as ''new normal''. Thus, the present results could also elucidate a pathway into adjusting biomarkers reference ranges personalized for individuals with SCI. A fact that is currently being explored by some research groups by creating an adjusted (i.e., age, gender, disability) normal reference ranges for laboratory values (such as the ongoing Swiss BioRef project). Lastly, it is clear that blood biomarkers assessment is of major clinical importance for diagnosis and prognosis, especially within populations at risk such as SCI, therefore, it is important to consider in a parallel approach the use of epigenetic biomarkers. In the CNS, emerging evidence has shown that epigenetic regulation plays a critical role in numerous pathological and physiological processes, such as proliferation, differentiation and regeneration. SCI comprises multiple epigenetic landmark alterations, identifying these could lead to the development of clinical solutions that promote the mechanisms of SCI recovery [83]. However, currently validation of epigenetic biomarkers specific for SCI is not available. Further research should focus on their application in SCI and its related secondary health conditions.
Abbreviations
- ABI:
-
Able-bodied individuals
- ACTH:
-
Adrenocorticotropic hormone
- BMD:
-
Bone mineral density
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CVD:
-
Cardio vascular disease
- FSH:
-
Follicle-stimulating hormone
- GH:
-
Growth hormone
- HbA1c:
-
Hemoglobin A1c (glycosylated hemoglobin)
- HPA:
-
Hypothalamus-pituitary-adrenal
- hsCRP:
-
High sensitivity c-reactive protein
- IGF-1:
-
Insulin-like growth factor 1
- IL-6:
-
Interleukin-6
- LH:
-
Luteinizing hormone
- SHBG:
-
Sex hormone-binding globulin
- PTH:
-
Parathyroid hormone
- SCI:
-
Spinal cord injury
- SI:
-
International system of units
- TNF-alpha:
-
Tumor necrosis factor alpha
- TSH:
-
Thyroid-stimulating hormone
- T3:
-
Triiodothyronine
- WMD:
-
Weighted mean difference
- 2 h-PG:
-
2 hour postload glucose
References
World Health Organization and International Spinal Cord Society. International perspectives on spinal cord injury. Geneva: WHO Press. 2013.
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3(1):17018.
Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–6.
Merritt CH, Taylor MA, Yelton CJ, Ray SK. Economic impact of traumatic spinal cord injuries in the United States. Neuroimmunol Neuroinflamm. 2019.
DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80(11):1411–9.
Chamberlain JD, Meier S, Mader L, von Groote PM, Brinkhof MW. Mortality and longevity after a spinal cord injury: systematic review and meta-analysis. Neuroepidemiology. 2015;44(3):182–98.
Jensen MP, Truitt AR, Schomer KG, Yorkston KM, Baylor C, Molton IR. Frequency and age effects of secondary health conditions in individuals with spinal cord injury: a scoping review. Spinal Cord. 2013;51(12):882–92.
Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile – Part I. J Spinal Cord Med. 2014;37(6):693–702.
Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr., Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol (1985). 2003;95(6):2398–407.
Garcia-Arguello LY, O’Horo JC, Farrell A, Blakney R, Sohail MR, Evans CT, et al. Infections in the spinal cord-injured population: A systematic review. Spinal Cord. 2017;55(6):526–34.
Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc. 2007;106(11):919–28.
Rönnbäck C, Hansson E. The importance and control of low-grade inflammation due to damage of cellular barrier systems that may lead to systemic inflammation. Front Neurol. 2019;10.
Sun X, Jones ZB, Chen X-m, Zhou L, So K-F, Ren Y. Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship. J Neuroinflamm. 2016;13(1):260.
Sakakibara BM, Hitzig SL, Miller WC, Eng JJ, the SRT. An evidence-based review on the influence of aging with a spinal cord injury on subjective quality of life. Spinal Cord. 2012;50(8):570–8.
Charlifue S, Jha A, Lammertse D. Aging with spinal cord injury. Phys Med Rehabil Clin N Am. 2010;21(2):383–402.
Hitzig SL, Eng JJ, Miller WC, Sakakibara BM, the SRT. An evidence-based review of aging of the body systems following spinal cord injury. Spinal Cord. 2011;49(6):684–701.
Stenholm S, Maggio M, Lauretani F, Bandinelli S, Ceda GP, Di Iorio A, et al. Anabolic and catabolic biomarkers as predictors of muscle strength decline: the InCHIANTI study. Rejuvenation Res. 2010;13(1):3–11.
Meng Y, Wu H, Yang Y, Du H, Xia Y, Guo X, et al. Relationship of anabolic and catabolic biomarkers with muscle strength and physical performance in older adults: a population-based cross-sectional study. BMC Musculoskelet Disord. 2015;16(1):202.
Pavlicek D, Krebs J, Capossela S, Bertolo A, Engelhardt B, Pannek J, et al. Immunosenescence in persons with spinal cord injury in relation to urinary tract infections -a cross-sectional study. Immun Ageing. 2017;14:22-.
Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53(1):14–8.
Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med. 2014;37(1):32–9.
Naderi AR, Safarinejad MR. Endocrine profiles and semen quality in spinal cord injured men. Clin Endocrinol. 2003;58(2):177–84.
Liang H, Mojtahedi MC, Chen D, Braunschweig CL. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil. 2008;89(1):36–41.
Radulovic M, Bauman WA, Wecht JM, Lafountaine M, Kahn N, Hobson J, et al. Biomarkers of inflammation in persons with chronic tetraplegia. J Breath Res. 2015;9(3):036001.
Gaspar AP, Brandão CMA, Lazaretti-Castro M. Bone mass and hormone analysis in patients with spinal cord injury: Evidence for a gonadal axis disruption. J Clin Endocrinol Metab. 2014;99(12):4649–55.
Celik B, Sahin A, Caglar N, Erhan B, Gunduz B, Gultekin O, et al. Sex hormone levels and functional outcomes: A controlled study of patients with spinal cord injury compared with healthy subjects. Am J Phys Med Rehabil. 2007;86(10):784–90.
Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49–60.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Ferguson DWS. Blood chemistry and CBC analysis. Jacksonville: Bear Mountain Pub. 2002.
Boehl G, Raguindin P, Valido E, Bertolo A, Fränkl G, Itodo O, et al. Data from: Inflammatory markers and hormones in individuals with spinal cord injury: A systematic review and meta-analysis. BORISDeposited 5 January 2022.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2013. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
van Assendelft OW, Mook GA, Zijlstra WG. International System of Units (SI) in physiology. Pflugers Arch. 1973;339(4):265–72.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86(2):312–7.
Gilbert O, Croffoot JR, Taylor AJ, Nash M, Schomer K, Groah S. Serum lipid concentrations among persons with spinal cord injury - a systematic review and meta-analysis of the literature. Atherosclerosis. 2014;232(2):305–12.
Kouda K, Furusawa K, Sugiyama H, Sumiya T, Ito T, Tajima F, et al. Does 20-min arm crank ergometer exercise increase plasma interleukin-6 in individuals with cervical spinal cord injury? Eur J Appl Physiol. 2012;112(2):597–604.
Gater DR, Farkas GJ, Berg AS, Castillo C. Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med. 2019;42(1):86–93.
Shojaei MH, Alavinia SM, Craven BC. Management of obesity after spinal cord injury: a systematic review. J Spinal Cord Med. 2017;40(6):783–94.
Knight ML. The application of high-sensitivity c-reactive protein in clinical practice: A 2015 update. US Pharmacist. 2015;40:50–3.
Fatima G, Sharma VP, Verma NS. Circadian variations in melatonin and cortisol in patients with cervical spinal cord injury. Spinal Cord. 2016;54(5):364–7.
Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS. Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med. 2000;23(2):129–35.
Leicht CA, Goosey-Tolfrey VL, Bishop NC. Spinal cord injury: known and possible influences on the immune response to exercise. Exerc Immunol Rev. 2013;19:144–63.
Clark MJ, Schopp LH, Mazurek MO, Zaniletti I, Lammy AB, Martin TA, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. 2008;87(9):758–67.
Safarinejad MR. Level of injury and hormone profiles in spinal cord-injured men. Urology. 2001;58(5):671–6.
Barbonetti A, Vassallo MRC, Pacca F, Cavallo F, Costanzo M, Felzani G, et al. Correlates of low testosterone in men with chronic spinal cord injury. Andrology. 2014;2(5):721–8.
Sullivan SD, Nash MS, Tefera E, Tinsley E, Blackman MR, Groah S. Prevalence and etiology of hypogonadism in young men with chronic spinal cord injury: A cross-sectional analysis from two university-based rehabilitation centers. PM&R. 2017;9(8):751–60.
Vitale G, Cesari M, Mari D. Aging of the endocrine system and its potential impact on sarcopenia. Eur J Intern Med. 2016;35:10–5.
Kamwa V, Welch C, Hassan-Smith ZK. The endocrinology of sarcopenia and frailty. Minerva Endocrinol. 2021;46(4):453–68.
Pelletier CA, Miyatani M, Giangregorio L, Craven BC. Sarcopenic obesity in adults with spinal cord injury: A cross-sectional study. Arch Phys Med Rehabil. 2016;97(11):1931–7.
Singh R, Rohilla RK, Saini G, Kaur K. Longitudinal study of body composition in spinal cord injury patients. Indian J Orthop. 2014;48(2):168–77.
Rabadi MH, Aston CE. Compare serum creatinine versus Renal 99mTc-DTPA scan determined glomerular filtration rates in veterans with traumatic spinal cord injury and meurogenic bladder. J Spinal Cord Med. 2016;39(6):638–44.
Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol. 2012;187(2):391–7.
Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44(12):1612–6.
Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Vitamin D, parathormone, and calcitonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil. 1994;75(7):766–9.
Flueck JL, Perret C. Vitamin D deficiency in individuals with a spinal cord injury: A literature review. Spinal Cord. 2017;55(5):428–34.
Lussi C, Frotzler A, Jenny A, Schaefer DJ, Kressig RW, Scheel-Sailer A. Nutritional blood parameters and nutritional risk screening in patients with spinal cord injury and deep pressure ulcer—a retrospective chart analysis. Spinal Cord. 2018;56(2):168–75.
Zhou XJ, Vaziri ND, Segal JL, Winer RL, Eltorai I, Brunnemann SR. Effects of chronic spinal cord injury and pressure ulcer on 25(OH)-vitamin D levels. J Am Paraplegia Soc. 1993;16(1):9–13.
Laird E, Ward M, McSorley E, Strain JJ, Wallace J. Vitamin D and bone health: potential mechanisms. Nutrients. 2010;2(7):693–724.
Kaya K, Aybay C, Ozel S, Kutay N, Gokkaya O. Evaluation of bone mineral density in patients with spinal cord injury. J Spinal Cord Med. 2006;29(4):396–401.
Gifre L, Vidal J, Carrasco JL, Muxi A, Portell E, Monegal A, et al. Risk factors for the development of osteoporosis after spinal cord injury. A 12-month follow-up study. Osteop Int. 2015;26(9):2273–80.
Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. 2017;85:78–97.
Huang TS, Wang YH, Lien IN. Suppression of the hypothalamus-pituitary somatotrope axis in men with spinal cord injuries. Metabolism. 1995;44(9):1116–20.
Bauman WA, Spungen AM, Wang J, Pierson RN Jr, Schwartz E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am J Physiol Endocrinol Metab. 2006;290(6):E1098–103.
Bauman WA, Spungen AM, Zhong YG, Mobbs CV. Plasma leptin is directly related to body adiposity in subjects with spinal cord injury. Horm Metab Res. 1996;28(12):732–6.
Latifi S, Koushki D, Norouzi Javidan A, Matin M, Sabour H. Changes of leptin concentration in plasma in patients with spinal cord injury: a meta-analysis. Spinal Cord. 2013;51(10):728–31.
LaVela SL, Evans CT, Prohaska TR, Miskevics S, Ganesh SP, Weaver FM. Males aging with a spinal cord injury: Prevalence of cardiovascular and metabolic conditions. Arch Phys Med Rehabil. 2012;93(1):90–5.
Farkas GJ, Pitot MA, Gater DR Jr. A systematic review of the accuracy of estimated and measured resting metabolic rate in chronic spinal cord injury. Int J Sport Nutr Exerc Metab. 2019;29(5):548–58.
Yarar-Fisher C, Li J, McLain A, Gower B, Oster R, Morrow C. Utilizing a low-carbohydrate/high-protein diet to improve metabolic health in individuals with spinal cord injury (DISH): study protocol for a randomized controlled trial. Trials. 2019;20(1):466.
Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20–3.
O’Reilly MW, Glisic M, Kumarendran B, Subramanian A, Manolopoulos KN, Tahrani AA, et al. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol. 2019;90(1):145–54.
Lu J, He J, Li M, Tang X, Hu R, Shi L, et al. Predictive value of fasting glucose, postload glucose, and hemoglobin a1c on risk of diabetes and complications in Chinese adults. Diabetes Care. 2019;42(8):1539–48.
Fan X-J, Yu H, Ren J. Homeostasis and compensatory homeostasis: bridging western medicine and traditional Chinese medicine. Curr Cardiol Rev. 2011;7(1):43–6.
Erlandsen EJ, Hansen RM, Randers E, Petersen LE, Abrahamsen J, Johannesen IL. Estimating the glomerular filtration rate using serum cystatin C levels in patients with spinal cord injuries. Spinal Cord. 2012;50(10):778–83.
Corvino V, Di Maria V, Marchese E, Lattanzi W, Biamonte F, Michetti F, et al. Estrogen administration modulates hippocampal GABAergic subpopulations in the hippocampus of trimethyltin-treated rats. Front Cell Neurosci. 2015;9:433.
Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29.
Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci U S A. 2003;100(2):703–8.
Raguindin PF, Muka T, Glisic M. Sex and gender gap in spinal cord injury research: Focus on cardiometabolic diseases. A mini review Maturitas. 2021;147:14–8.
Whalley HK. Experience of rehabilitation following spinal cord injury: a meta-synthesis of qualitative findings. Spinal Cord. 2007;45(4):260–74.
Ong B, Wilson JR, Henzel MK. Management of the patient with chronic spinal cord injury. Medical Clinics. 2020;104(2):263–78.
Zhang B-Y, Chang P-Y, Zhu Q-S, Zhu Y-H, Saijilafu. Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury. Neural Regen Res. 2020;15(9):1613–22.
Invernizzi M, Carda S, Rizzi M, Grana E, Squarzanti DF, Cisari C, et al. Evaluation of serum myostatin and sclerostin levels in chronic spinal cord injured patients. Spinal Cord. 2015;53(8):615–20.
Iversen PO, Groot PD, Hjeltnes N, Andersen TO, Mowinckel MC, Sandset PM. Impaired circadian variations of haemostatic and fibrinolytic parameters in tetraplegia. Br J Haematol. 2002;119(4):1011–6.
Matos-Souza JR, Pithon KR, Ozahata TM, Oliveira RT, Teo FH, Blotta MH, et al. Subclinical atherosclerosis is related to injury level but not to inflammatory parameters in spinal cord injury subjects. Spinal Cord. 2010;48(10):740–4.
Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87(3):600–7.
Schreiber R, Paim LR, de Rossi G, Matos-Souza JR, Costa ESAA, Nogueira CD, et al. Reduced Sympathetic Stimulus and Angiotensin 1–7 Are Related to Diastolic Dysfunction in Spinal Cord-Injured Subjects. J Neurotrauma. 2017;34(15):2323–8.
La Favor JD, Hollis BC, Mokshagundam SL, Olive JL. Serum hsCRP and visfatin are elevated and correlate to carotid arterial stiffness in spinal cord-injured subjects. Spinal Cord. 2011;49(9):961–6.
Lieberman J, Goff D Jr, Hammond F, Schreiner P, James Norton H, Dulin M, et al. Dietary intake relative to cardiovascular disease risk factors in individuals with chronic spinal cord injury: a pilot study. Top Spinal Cord Inj Rehabil. 2014;20(2):127–36.
Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88(11):1384–93.
Zhang C, Jing YL, Zhang WH, Zhang J, Yang ML, Du LJ, et al. Dysbiosis of gut microbiota is associated with serum lipid profiles in male patients with chronic traumatic cervical spinal cord injury. Am J Transl Res. 2019;11(8):4817.
Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749–56.
Jones LM, Legge M. Plasma fatty acids as markers for desaturase and elongase activities in spinal cord injured males. J Spinal Cord Med. 2019;42(2):163–70.
Mojtahedi MC, Valentine RJ, Arngrimsson SA, Wilund KR, Evans EM. The association between regional body composition and metabolic outcomes in athletes with spinal cord injury. Spinal Cord. 2008;46(3):192–7.
Dearwater SR, LaPorte RE, Robertson RJ, Brenes G, Adams LL, Becker D. Activity in the spinal cord-injured patient: an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc. 1986;18(5):541–4.
Hjeltnes N, De Groot P, Birkeland KI, Falch JA, Iversen PO. Tetraplegic subjects have hyperleptinaemia with marked circadian variation. Clin Endocrinol (Oxf). 2005;62(2):223–7.
Jeon JY, Harber VJ, Steadward RD. Leptin response to short-term fasting in sympathectomized men: role of the SNS. Am J Physiol Endocrinol Metab. 2003;284(3):E634–40.
Jones LM, Legge M, Goulding A. Factor analysis of the metabolic syndrome in spinal cord-injured men. Metabolism. 2004;53(10):1372–7.
Li J, Hunter GR, Chen Y, McLain A, Smith DL, Yarar-Fisher C. Differences in glucose metabolism among women with spinal cord injury may not be fully explained by variations in body composition. Arch Phys Med Rehabil. 2019;100(6):1061–7 e1.
Nelson MD, Widman LM, Abresch RT, Stanhope K, Havel PJ, Styne DM, et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30 Suppl 1(SUPPL. 1):S127–39.
Demirel S, Demirel G, Tukek T, Erk O, Yilmaz H. Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord. 2001;39(3):134–8.
Liang H, Chen D, Wang Y, Rimmer JH, Braunschweig CL. Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil. 2007;88(9):1198–204.
Akbal A, Kurtaran A, Gurcan A, Selcuk B, Batgi H, Akyuz M, et al. P-wave and QT dispersion in spinal cord injury. Intern Med. 2014;53(15):1607–11.
Akbal A, Kurtaran A, Selcuk B, Akyuz M. H-FABP, cardiovascular risk factors, and functional status in asymptomatic spinal cord injury patients. Herz. 2013;38(6):629–35.
Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, et al. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. 2008;46(7):494–9.
Paim LR, Schreiber R, Matos-Souza JR, Silva AA, Campos LF, Azevedo ER, et al. Oxidized low-density lipoprotein, matrix-metalloproteinase-8 and carotid atherosclerosis in spinal cord injured subjects. Atherosclerosis. 2013;231(2):341–5.
Zhang C, Zhang W, Zhang J, Jing Y, Yang M, Du L, et al. Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury. J Transl Med. 2018;16(1):353.
Burr RG, Chem C, Nuseibeh I. Creatinine, calcium, citrate and acid-base in spinal cord injured patients. Paraplegia. 1993;31(11):742–50.
Finsen V, Indredavik B, Fougner KJ. Bone mineral and hormone status in paraplegics. Paraplegia. 1992;30(5):343–7.
Gaspar AP, Brandao CM, Lazaretti-Castro M. Bone mass and hormone analysis in patients with spinal cord injury: evidence for a gonadal axis disruption. J Clin Endocrinol Metab. 2014;99(12):4649–55.
Bartoletti R, Gavazzi A, Cai T, Mondaini N, Morelli A, Del Popolo G, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142–8.
Hvarness H, Jakobsen H, Biering-Sorensen F. Men with spinal cord injury have a smaller prostate than men without. Scand J Urol Nephrol. 2007;41(2):120–3.
Ibrahim E, Aballa TC, Roudebush WE, Lynne CM, Brackett NL. Inhibin B is lower and anti-Mullerian hormone is similar in serum of men with spinal cord injuries compared to controls. Syst Biol Reprod Med. 2015;61(2):72–7.
Mathian B, Pallant A, Pilonchéry G, Charvier K, Patricot MC. L’Inhibine B chez les blessés radiculo-médullaires. Résultats préliminaires Andrologie. 1999;9(3):387–93.
Cheol SJ, Il PC, Wook RD, Joongson C, Eun KJ, Chul JS, et al. Sex Hormone Profiles in Spinal Cord-Injured Patients. Ann Rehabil Med. 2004;28(3):226–31.
Tsitouras PD, Zhong YG, Spungen AM, Bauman WA. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res. 1995;27(6):287–92.
Arija-Blazquez A, Ceruelo-Abajo S, Diaz-Merino MS, Godino-Duran JA, Martinez-Dhier L, Florensa-Vila J. Time-course response in serum markers of bone turnover to a single-bout of electrical stimulation in patients with recent spinal cord injury. Eur J Appl Physiol. 2013;113(1):89–97.
Huang TS, Wang YH, Lee SH, Lai JS. Impaired hypothalamus-pituitary-adrenal axis in men with spinal cord injuries. Am J Phys Med Rehabil. 1998;77(2):108–12.
Kikuchi TA, Skowsky WR, El-Toraei I, Swerdloff R. The pituitary-gonadal axis in spinal cord injury. Fertil Steril. 1976;27(10):1142–5.
Nance PW, Shears AH, Givner ML, Nance DM. Gonadal regulation in men with flaccid paraplegia. Arch Phys Med Rehabil. 1985;66(11):757–9.
Gokkaya CS. Prostate-specific antigen and its derivatives in spinal cord injured patients; a case-control study. J Clin Anal Med. 2013;4(6).
Gökkaya CS, Öztekin ÇV, Demirdal O, Gökkaya NK, Özden C, Uçan H, et al. Prostate-specific antigen and its derivatives in spinal cord injured patients; a case-control study. J Clin Anal Med. 2013;4(6).
Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77(2):371–8.
Cheville AL, Kirshblum SC. Thyroid hormone changes in chronic spinal cord injury. J Spinal Cord Med. 1995;18(4):227–32.
Formisano R, Grelli S, Matteucci C, Santilli VV, Vinicola VV, Scivoletto G, et al. Immunological and endocrinological disturbances in patients after prolonged coma following head injury. Eur J Neurol. 1998;5(2):151–8.
Campagnolo DI, Bartlett JA, Chatterton R Jr, Keller SE. Adrenal and pituitary hormone patterns after spinal cord injury. Am J Phys Med Rehabil. 1999;78(4):361–6.
Munakata M, Kameyama J, Kanazawa M, Nunokawa T, Moriai N, Yoshinaga K. Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: simultaneous evaluation of autonomic nervous activity and physical activity. J Hypertens. 1997;15(12 Pt 2):1745–9.
Sica DA, Midha M, Aronoff G, Bergen G. Atrial natriuretic factor in spinal cord injury. Arch Phys Med Rehabil. 1993;74(9):969–72.
Maimoun L, Puech AM, Manetta J, Badiou S, Paris F, Ohanna F, et al. Circulating leptin concentrations can be used as a surrogate marker of fat mass in acute spinal cord injury patients. Metabolism. 2004;53(8):989–94.
Wang YH, Huang TS, Liang HW, Su TC, Chen SY, Wang TD. Fasting serum levels of adiponectin, ghrelin, and leptin in men with spinal cord injury. Arch Phys Med Rehabil. 2005;86(10):1964–8.
Kostovski E, Iversen PO, Birkeland K, Torjesen PA, Hjeltnes N. Decreased levels of testosterone and gonadotrophins in men with long-standing tetraplegia. Spinal Cord. 2008;46(8):559–64.
Kaya K, Ünsal S, Ordu Gökkaya NK, Aybay C, Özel S. The evaluation of bone mineral density in traumatic cervical spinal cord injury patients. Journal of Rheumatology and Medical Rehabilitation. 2004;15(2):94–101.
Ito T, Higuchi Y, Banno H, et al. Blood volume in patients with cervical spinal cord injury. J Phys Ther Sci. 2004;16(2):81–4. https://doi.org/10.1589/jpts.16.81.
Shin JC, Park CI, Rha DW, Chon J, Kim JE, Jeon SC, Jung TH. Sex hormone profiles in spinal cord-injured patients. J Korean Acad Rehabil Med. 2004;28:226–31.
Funding
Open access funding provided by University of Bern. Gabriela Boehl and Alexander Leichtle are funded by Swiss Personalized Health Network (SPHN), “Swiss BioRef” (grant 2018DEV22), Jivko Stoyanov is funded by SPHN and Swiss Paraplegic Foundation. Peter Francis Raguindin, Ezra Valido and Oche Adam Itodo have received Marie Sklodowska-Curie funding from the European Union Horizon 2020 program (grant No. 801076) through the Global Ph.D. Fellowship Program in Public Health Sciences of the Swiss Schools of Public Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boehl, G., Raguindin, P.F., Valido, E. et al. Endocrinological and inflammatory markers in individuals with spinal cord injury: A systematic review and meta-analysis. Rev Endocr Metab Disord 23, 1035–1050 (2022). https://doi.org/10.1007/s11154-022-09742-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09742-9