Abstract
Type 1 diabetes mellitus imposes a significant burden of complications and mortality, despite important advances in treatment: subjects affected by this disease have also a worse quality of life-related to disease management. To overcome these challenges, different new approaches have been proposed, such as new insulin formulations or innovative devices. The introduction of insulin pumps allows a more physiological insulin administration with a reduction of HbA1c level and hypoglycemic risk. New continuous glucose monitoring systems with better accuracy have allowed, not only better glucose control, but also the improvement of the quality of life. Integration of these devices with control algorithms brought to the creation of the first artificial pancreas, able to independently gain metabolic control without the risk of hypo- and hyperglycemic crisis. This approach has revolutionized the management of diabetes both in terms of quality of life and glucose control. However, complete independence from exogenous insulin will be obtained only by biological approaches that foresee the replacement of functional beta cells obtained from stem cells: this will be a major challenge but the biggest hope for the subjects with type 1 diabetes. In this review, we will outline the current scenario of innovative diabetes management both from a technological and biological point of view, and we will also forecast some cutting-edge approaches to reduce the challenges that hamper the definitive cure of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the disruption of pancreatic beta cells: this leads to a progressive reduction of insulin secretion and subsequent hyperglycemia, along with lipid and protein metabolism derangements. The DCCT-EDIC study showed that hyperglycemia, in type 1 diabetes, is associated with micro-and macrovascular complications and increased mortality[1,2,3]. To survive, subjects with type 1 diabetes must rely on exogenously injected insulin in subcutaneous tissue: this ensures adequate basal and prandial insulin concentrations to recreate physiological insulin profiles to avoid ketoacidosis and hyperglycemia-related complications [4]. The most relevant limiting factor for achieving good glycemic levels is hypoglycemia, defined as glycemic values lower than 70 mg/dl (3.9 mmol/L), determined by a discrepancy between insulin administration and carbohydrate (CHO) intake [5,6,7,8]. Hypoglycemia impacts the quality of life and leads to acute complications like seizures and coma, and, potentially, to a heart attack. The fear of hypoglycemia leads the patients to accept higher glycemic values, making more difficult the achievement of a good metabolic control [9,10,11]. To inject suitable insulin doses T1D subjects (T1Ds) must: 1. monitor their glucose values several times/day (self-monitoring of blood glucose, SMBG), 2. know the exact amount of CHO in their diet, 3. calculate the correct ratio between CHO taken and insulin to administer (I: CHO ratio), 4. estimate the impact of physical activity, illness, and stressful episodes. All these commitments lead T1Ds to face numerous daily decisions with an important deterioration of quality of life (QoL) [12,13,14]. Regrettably, the subcutaneous administration of insulin is non-physiological since the portal-to-periphery ratio of hormone concentrations is reversed leading to a relative peripheral overinsulinization and frequently unmatched insulin levels for the prevalent glucose concentrations. To overcome this problem, new insulin with more physiological pharmacodynamic have been introduced in the market; basal insulin analogs with longer duration (degludec, glargine U300), demonstrated their efficacy in maintaining metabolic control without hypoglycemia, especially during the night. [15,16,17,18,19]. On the other hand, new ultra-rapid prandial insulin analogs lead to better postprandial glycemic control reducing hyperglycemia in the early post-prandial phase. In a recent meta-analysis, faster aspart demonstrated efficacy in T1Ds in terms of reduction of HbA1c without increasing the overall hypoglycemic episodes. [20, 21]. Furthermore, insulin pumps (continuous subcutaneous insulin infusion, CSII) could ensure a more physiological approach [22]. Beyond insulin, other drugs have been proposed for the management of type 1 diabetes, in association with insulin [23]. In particular, sodium–glucose co-transporter-2 (SGLT2) inhibitors can reduce the HbA1c along with weight loss and reduction of daily insulin dose [24], especially in overweight subjects: this paved the way to the approval for dapagliflozin use in overweight (body mass index > 27 kg/m2) T1Ds in association with insulin in several countries. However, it is important to underline the potential risk of ketoacidosis associated with the use of these drugs, especially when the insulin dose is excessively down-titrated [25, 26]. In addition to SGLT2 inhibitors, other drugs approved in type 2 diabetes have been evaluated for T1Ds. Metformin demonstrated a reduction in BMI and insulin requirements, with no clear effects on HbA1c [27]. Glucagon-like peptide 1 receptor agonists (GLP-1RA), used for the treatment of T2D and obesity, demonstrated potential efficacy in clinical trials also in T1Ds when adjunct to insulin; a recent meta-analysis confirmed that GLP-1RA improve glycemic control, reduce severe hypoglycemia, body weight, and insulin requirements [28].
The monitoring of glucose levels has also been improved with the introduction in the market of smaller, more accurate, glucose monitoring systems that allow patients with T1Ds to visualize every 1 to 5 min their glucose values [29]. Despite these innovations, people with type 1 diabetes still have a reduced life expectancy [30], with an increased risk of both macro-and microvascular complications and a worse quality of life compared to the non-diabetic population [31]. To optimize diabetes control, three main fields have been investigated: pharmacological, technological, and biological approaches. From a pharmacological standpoint, new insulin formulations have undoubtedly allowed higher efficacy, safety, and flexibility in the management of diabetes. The technological approach has allowed more sophisticated insulin pumps, sensors, glucometers, capable of simplifying, and improving diabetes management. Technology has also helped the management of diabetes thanks to easier data recording and safer data sharing between clinicians, patients, and caregivers. The biological approach aims to completely replace the production of insulin: in the last decades, either pancreas or beta-cell transplantation has dramatically improved as well as immunosuppression so that beta-cell replacement can now be considered an option to cure T1Ds. Regrettably, this type of approach is limited by the lack of organs and by the exposure of subjects to the consequence of immunosuppressive therapy, so that researchers are actively seeking to create new beta cell source from stem cells, to guarantee insulin production without the immunosuppressive therapy. This review describes innovative technological and biological approaches for diabetes management, highlighting future strategies that could be developed to reduce the burden related to diabetes and maybe to find a cure.
2 Technology innovation
In recent years technology has revolutionized the management of diabetes: the technological approach is based on the use of insulin pumps and sensors for continuous glucose monitoring, and on the possibility to integrate these 2 systems to create a device capable of autonomously modifying the administration of insulin according to the values detected by the sensor, thus creating the so-called artificial pancreas or closed-loop system.
3 State of the art
3.1 Insulin pump
Since their introduction in the 70 s, these devices have undergone important improvement, both in terms of portability and functionality. Insulin pumps allow the continuous administration of rapid insulin analogs, infused at different pre-programmable basal rates that mimick the secretion of physiological hormone response. Furthermore, the administration of meal insulin boluses can also be protracted to allow a better insulinization in response to meals enriched in protein and fat that have a significantly slower absorption. CSII leads to an improvement in glycemic control and reduction of hypoglycemia. Several studies demonstrated a statistically significant reduction of both HBA1c and hypoglycemic events in patients on CSII (Table 1) [32,33,34,35,36,37,38]. In a meta-analysis of the available randomized controlled studies (RCT), Pickup and colleagues showed that CSII reduces HBA1c by 0.21% as compared to multiple daily injection (MDI) therapy [33]. Similarly, in 2010 Monami and colleagues reported a reduction of HbA1c of 0.3% [34]. All meta-analysis compared CSII efficacy vs glargine or NPH insulin basal but relatively fewer data are available on CSII efficacy vs. MDI performed with new basal analogs. However, a more recent meta-analysis demonstrated superior efficacy of CSII in reducing HbA1c also in trials in which a rapid-acting analog was used; the advantage of CSII vs. MDI was smaller than that observed in trials using regular human insulin [35]. Data on hypoglycemic events are less clear: a similar hypoglycemic risk between CSII and MDI has been reported. Notably, it must be also acknowledged that there are insufficient data about efficacy in children [36]. CSII requires greater management skills and commitment than MDI therapy but, at the same time, allows greater flexibility in controlling the daily activities, and this leads to an improvement of the patients’ quality of life. Several trials demonstrated a better acceptance of this approach with a parallel reduction of the burden related to diabetes [39, 40]. The risks associated with ketoacidosis secondary to the occlusion of the infusion set were reported to be minimal [41].
Last but not least, CSII-based treatment is associated with a reduction of mortality and complications [42], as reported by the Swedish register that evaluated more than 18,000 T1Ds treated with CSII or MDI [43]. Reduction of mortality, especially related to cardiovascular events, could be related to lower hypoglycemic events and more stable glucose values.
Another approach for insulin infusion is represented by continuous intraperitoneal insulin infusion (CIPII). CIPII provides an alternative insulin administration, through an implantable pump, allowing a more physiological delivery since insulin is absorbed through the portal system, thus mimicking the physiological condition [44]. The need for surgery and the costs limit this option for T1Ds who fail to achieve satisfactory glycemic control with other treatments. Several studies demonstrated CIPII efficacy when compared to CSII in term of HbA1c and severe hypoglycemia reduction and treatment satisfaction [45, 46]
3.2 Continuous glucose monitoring (CGM)
Continuous glucose monitoring (CGM) represents an awesome improvement in the possibilities of monitoring the glucose levels: these devices continuously detect the glucose concentrations in subcutaneous tissue thanks to small sensors that can be replaced every 7–14 days. CGM systems can be divided into real-time (rt-CGM) and intermittently scanned (is-CGM) devices. Rt-CGM provides real-time glucose values, allowing the patient to view, not only the glucose levels but also the future trends prediction and past trends on both the receiver or on a smartphone app which provides appropriate alerts for both high and low glucose readings: with rt-CGM the patient is aware when a given glycemic threshold is exceeded or when it is about to be exceeded [47,48,49]. These devices demonstrated superiority in their efficacy over SMBG in terms of HbA1c reduction, glucose variability, and hypoglycemia reduction in subjects treated either with CSII or MDI [50,51,52,53,54,55,56,57,58,59,60,61], as shown in Table 2. Unfortunately, their use may be intermittent for weekly sensor replacement [62]. Over the last years, their accuracy has been improved, and some of them have been approved for non-adjunctive use, allowing patients with T1D to adopt decisions regarding their insulin therapy without the need for capillary glucose control [63]. Some devices need calibration vs. capillary glucose to ensure adequate accuracy, but now devices factory-calibrated are available [64]. An implantable subcutaneous sensor of 180 days duration has been recently introduced: this approach avoids the need for weekly sensor replacement with similar efficacy in terms of metabolic control [65, 66]. Is-CGM or flash glucose monitoring system (FGM), on the other hand, does not provide alarms and allows the patient to view glycemic values and trends when the patient scans the sensor through the reader or mobile phone. Is-CGM has proven its effectiveness in improving glycemic control and reducing hypoglycemic risk [67,68,69]. Recently, a new version of is-CGM provided optional alerts for high and/or low glucose levels, thus advising T1Ds to perform a scan to evaluate the actual glucose level. All these devices lead to an improvement in QoL when compared to SMBG [52, 70, 71], due to the possibility to visualize data continuously without the need for finger sticks.
Both Rt-CGM and is-CGM provide predictions of the glucose levels based on previous glucose readings: these data could be used by T1Ds to adjust insulin correction or prandial boluses and CHO intake. This represents additional support in the management of T1DM, in particular at mealtime, when multiple parameters such as insulin: carbohydrate ratio, glucose target, and correction factor should be taken into account. Several recommendations have been published regarding trend arrow management: as an example, a percentage or fixed values could be added or subtracted to a prandial insulin bolus based on the rate of glucose changes [72, 73]. Recently, more personalized approaches have been introduced based on insulin sensitivity factors and different baseline glucose levels. [74, 75]
The availability of these devices has changed the metric to assess glucose control: the possibility to visualize daily glucose profiles have shifted the gold standard parameter for metabolic control HBA1c to parameters such as time in target range (TIR), time spent between 70 and 180 mg/dl, which have updated the goals to be achieved by the patients [76]. Other parameters complementary to TIR are time spent with glucose values below 70 mg/dl, time below range (TBR), and time spent above 180 mg/dl, time above range (TAR). These parameters have some limits, related to the lack of an established standard for glucose measurement with CGM: as suggested by several authors, TIR should be regarded as a complement to HbA1c [77]. Indeed HbA1c values have been considered over the last decades the parameter that better correlates with clinical outcomes, even though additional evidence of a correlation between TIR and diabetes complications are emerging, both for micro and macrovascular complications [78,79,80].
3.3 Blood glucose meters (BGM)
Although the use of CGM is increasing, some T1Ds continue to use BGM to check their glucose values: it might be related to either the lack of CGM accuracy, or their cost and unacceptability [81, 82]. Several studies demonstrated the efficacy of BGM in reducing both HBA1c and the hypoglycemic events when tests are performed correctly, usually from six to ten times a day, even if the visualization of the glucose levels are intermittent [83]. BGM technology have been improved over the last years [84]. Accuracy of devices is crucial not only to correctly manage the disease but also to calibrate CGM; accuracy of BGM could be compared to the reference values of venous blood glucose [85]. New BGM could be connected to a smartphone app leading to a better patient’s engagement and to the possibility of sharing directly data with phisicians or caregivers [86]. Several devices also have other features, such an alarm to remind the subject to check her/his blood sugar, or a bolus calculator integrated into the BGM that simplifies the calculation of prandial bolus amount based on the subjects’ parameter [87].
3.4 Sensor augmented pump and first automatic systems
Given the superiority of the CSII over MDI and CGM over SMBG, the gold standard for the treatment of type 1 diabetes should be the combination of CSII with CGM, called Sensor Augmented Pump (SAP) Therapy. This combination is superior when compared to CSII + SMBG in terms of improving glycemic control and reducing hypoglycemia [51]. Nonetheless, subjects on SAP therapy in apparently good metabolic control spend several hours in both hypo and hyperglycemia, indicating that more precise approaches are required to obtain glycemic values comparable to those observed in subjects without diabetes [88]. For this reason, systems with automated modification in insulin administration based on the values detected by the sensor have been assessed. The first automated approach was dedicated to control hypoglycemia: the Low Glucose Suspend system (LGS) interrupts insulin infusion for a maximum of 2 h when a predetermined low glucose level is reached: this approach can reduce severe hypoglycemia, even if compared to SAP without LGS [89], especially in T1D at high risk of hypoglycemia or with reduced hypoglycemia awareness [90]. The second approach, a further step towards better management of diabetes, was achieved through the introduction of Predictive Low Glucose suspend (PLGS), capable of suspending the basal administration of insulin when hypoglycemia was predicted by the sensor with a further reduction of hypoglycemic risk [91,92,93]. In this context, real-life studies have shown the efficacy of this algorithm in improving metabolic control [94,95,96].
3.5 Artificial pancreas
The artificial pancreas (AP) or closed-loop control (CLC) system is a technology that allows the control of blood glucose concentrations in a completely automated manner. This device is comprised of an insulin pump, a CGM, and a control algorithm (CA) that automatically modifies insulin infusion according to prevailing glucose concentrations. Insulin infusion is therefore modified every few minutes based on new glucose values received by CGM: CLC increases insulin infusion when glucose values are increasing and decreases or suspends insulin infusion in case of significant reduction of glucose levels to minimize the risk of hypoglycemia (Fig. 1). Different models have been developed with different insulin pumps and different CGM and especially different CA, the “brain” of the system [97, 98]. In the last decade, several studies have assessed AP performances, initially in the inpatient setting [99, 100] to evaluate its safety and efficacy, then in patients’ real-life conditions to demonstrate their feasibility [101, 102]. All these trials established the superiority of AP compared to CSII or SAP, in terms of time spent in target, hypoglycemia reduction, HbA1c improvements, and acceptability by T1D subjects. Performances of AP were evaluated also in children and adolescents [103] and in pregnant women with T1D [104,105,106].
Artificial pancreas components, its limitations, and future perspectives: The algorithm modifies automatic insulin infusion throughout the insulin pump based on glucose values registered by CGM to optimize glucose control increasing time spent in a target (70–180 mg/dl). Challenges are related to insulin absorption that should be accelerated, CGM accuracy, and the need for calibration. To reduce the burden related to diabetes a full closed-loop control that minimizes the subject's intervention could completely automatize insulin therapy. FLCL: full closed-loop control, CGM: continuous glucose monitoring
These trials lead to the introduction of the first commercially available CLC system, MiniMed 670G (Medtronic MiniMed, Inc., Northridge, CA, USA): this device is called a Hybrid closed-loop (HCL) because subjects have to announce meal intake to avoid postprandial hyperglycemia [107, 108]. In a pivotal registration trial this device showed, in both adults and adolescents, its efficacy [109] with a reduction in HBA1c values (from 7.7% ± 0.8% to 7.1% ± 0.6% (P < 0.001) in adolescents, and from 7.3% ± 0.9% to 6.8% ± 0.6% (P < 0.001) in adults, and with a parallel increase of TIR (from 60.4% ± 10.9% to 67.2% ± 8.2% (P < 0.001) in adolescents and from 68.8% ± 11.9% to 73.8% ± 8.4% (P < 0.001) in adults. Similar results were confirmed also in the pediatric population from 7 to 13 years [110], which often has a more challenging glycemic control. The efficacy of the system is proportional to the time when CLC is active (auto mode) [111]. Recently, a randomized controlled trial [112] confirmed the efficacy of Minimed 670G during 26 weeks, with a reduction of HbA1c and an increase of the time spent in target when compared to standard therapy. In this trial, it has been demonstrated also an improvement of diabetes-specific quality of life, evaluated through validated questionnaires. Currently, Minimed 670G is approved for T1Ds older than 7 years old. Since it has been commercialized in 2017 in the US, real-world data have been published [113], confirming the efficacy of the device. Nevertheless, in 1 year follow up observational study of T1Ds who started 670G use, a reduction of Auto Mode over time was observed; 46% of users stopped auto mode after 1 year and only 32% of subjects have used auto mode for at least 70% of the time [114]. These data suggest that beyond the efficacy of the device, other details have to be considered: suspension of auto mode was related to alarms reported by devices and the need for sensor calibration. Other reasons are related to the unique glucose target available (120 mg/dl), not acceptable by subjects with tighter glycemic control, such as during pregnancy. Factory calibrated CGM could solve the glitches related to calibration but improvements in AP models are necessary to increase their time of use. For this reason, an enhanced version of 670G, called 780G, obtained the CE mark in June; this new version could dispense automated correction boluses, have different optional glucose targets, and other features to increase the utilization of Auto Mode [115, 116].
Other devices have been evaluated and authorized for commercial use such as Tandem Control IQ, which proved its efficacy with a sensor that needs no calibrations, by increasing time in the target (from 61 ± 17% at baseline to 71 ± 12% at the end of 6 months study period), by reducing HBA1c values (-0.33% in CLC group) and hypoglycemic events [117]. This AP model was also assessed in the pediatric population [118] during a winter camp and showed its efficacy also in this specific population and during physical activity (percent time within range was 66.4 ± 16.4 vs 53.9 ± 24.8% with P-value 0.01). Since this system is available in the US from the beginning of 2020, the first real-life data have been published, confirming results obtained in clinical trials with improvements also in psychosocial outcomes [119].
Other HCL systems either received or are waiting for approval, and will be commercialized in the next years. CamAPS FX, which uses an algorithm non installed on an insulin pump but on a smartphone that communicates with the pump and sensor, received a CE mark for 1 year, and different trials demonstrated its efficacy also in adolescents and children [120, 121]. Diabeloop algorithm is also installed in a smartphone, and communicate with CGM and patch pump, CSII system without a catheter. In a randomized crossover trial, an increase of 9.2% of the time spent in the target was observed using this AP [122], and performances were evaluated also in more challenging situations as meals and physical exercise [123]. The Omnipod Horizon system [124] uses a patch pump and both its safety and efficacy were demonstrated in both adults and pediatric T1Ds even also in an outpatient setting [125, 126]. CLCs equally allow a better QoL, by reducing the burden related to diabetes by demonstrating a significant reduction of the time spent in diabetes management [127, 128]. These results need to be confirmed in real life since the effectiveness of clinical trials in selected subjects could have impacted the results. Since 2013 it is active as a movement for the development of open-source diabetes management systems (Do-It-Yourself Artificial Pancreas Systems, DIY), with the scope of accelerating AP development and access. This group aims to create an “open source” artificial pancreas, sharing algorithms with personalized settings, and glucose targets. These algorithms can communicate with several existing devices via Bluetooth thus enabling the conception of personalized insulin pumps and CGM, thus overcoming the marked systems. There are no clinical trials that have tested these systems, but data set analysis and real-world data suggested an improvement in HBA1c values and time spent in target and amelioration of QoL [129]. The lack of evidence by RCT and the absence of regulation poses also obvious legal problems for users.
3.6 Further role of technology
Technology can simplify the management of diabetes: as an example, smart insulin pens with memory functions could record the insulin doses administered and transfer data via Bluetooth to dedicated apps [130]. Several smartphone apps for diabetes management have been developed, with the aim of help T1Ds to calculate insulin bolus, registered glucose data, track carbohydrate intake, or physical activity, with the possibility of sharing data on glycemic trends with clinicians. Also, CGM data could be managed with a smartphone app and shared in a cloud system, thus allowing also clinicians to visualize glucose values. This leads to the development of telemedicine methods which are tremendously useful when subjects can’t access the clinic, as recently occurred during Covid 19 pandemic [131, 132]. Similarly, data could be shared between T1Ds and caregivers, especially for example for children with T1D.
3.7 Future prospectives
3.7.1 Continuous glucose monitoring
Even if substantial advancements have been made in the field of glucose sensors in terms of accuracy and portability, they remain needle-based device with reduced acceptance, especially in childhood. For this reason, researchers are working on new projects based on non-invasive glucose monitoring using alternative body fluids [133]. For example, a wearable patch to measure glucose on sweat has been tested [134], even if the contamination of skin, the impact of physical activities, and related changes in sweat production may represent major problems to solve. The determination of glucose in tears has also be considered using a contact lens-based system [135]: this device appears to have an accuracy comparable to the commercialized CGM system. Also, salivary glucose concentration correlates with those in plasma [136], but challenges related to the interferences with food or bacteria in the mouth limit the development of these devices.
3.7.2 Artificial pancreas
The real-life data obtained during the first-year experience with 670G [114] suggest that, beyond the efficacy in glucose control, other features should be considered to optimize automatic system use. The possibility to rely on correction boluses and the reduction of alarms in the 780G model and factory calibrated devices (in the AP model that uses non-adjunctive sensors) could improve device acceptance. Future prospective in AP development foresees the possibility of creating a full CLC (FCLC) that does not need a subject’s interventions. The main challenges in FCLC development are related to the difficulties in managing postprandial control with no meal announcement and during physical activity. In 2008 Weinzimer and colleagues [137] compared an FCLC and an HCL in an inpatient setting in adolescents. They demonstrated that, although the 2 systems performed similarly in the overnight period, the postprandial phase was better managed by HCL with meal announcement and prandial bolus administration 15 min before a meal with a postprandial peak of 226 ± 51 mg/dl vs 194 ± 47 (p-value 0.04). Similar results were obtained in an inpatient setting by Forlenza et al. in both adults and adolescents who underwent AP session with announced and unannounced meals; They showed that the postprandial CGM average was significantly lower for announced than for unannounced meals (140.6 ± 35.0 vs. 197.8 ± 44.1 mg/dl, p < 0.001) [138]. Challenges in postprandial peak management with no meal announcement are related to relative delay in insulin absorption. No significant improvements were observed in the postprandial phase in FCLC using FasterAspart compared to AspArt [139], thus demonstrating that the insulin absorption limits the postprandial peak management in FCLC. It has been shown that intraperitoneal insulin infusion allowed better control in unannounced meals, with a reduction of time spent in hyperglycemia in the postprandial phase [140]. This approach is not feasible in the real life in the majority of T1Ds but suggests that a more physiological and rapid insulin administration may be a potential solution for the postprandial peak challenge. Another approach to control postprandial peak is the pramlintide association. Pramlintide is an analog of amylin, co-secreted with insulin and deficient in T1D, that delays gastric emptying and suppresses glucagon secretion. Use of subcutaneous Pramlintide in FCLC was associated with a reduction of the postprandial magnitude of glycemic excursion (88 ± 42 vs. 113 ± 32 mg/dL; P = 0.006) compared with CLC alone [141]. Another challenge is related to physical activity management [142]: with commercialized AP models, the strategy of establishing a pre-set of different higher glucose targets reduces the risk of hypoglycemia. Methods to communicate physical activity to algorithms have been investigated such as adding hearth rate signal, as a surrogate of physical activity, measured through a heart rate monitor [143]. This approach reduced hypoglycemic risk during exercise and increased time in the target range (81% vs. 75%;P = 0.2). The necessity to wear another device limits this approach in real life.
3.7.3 Bihormonal artificial pancreas
The main limitation to achieve better glycemic control is the hypoglycemic risk: to overcome this problem, a bihormonal approach could maximize the efficacy of AP in reducing the risk of hypoglycemia thanks to the co-administration of glucagon. A bihormonal pancreas (BP) is similar to AP and consists of a CA installed in a smartphone that communicates with CGM, insulin, and glucagon pump. Studies that evaluated the efficacy of BP had a rather shorter duration as compared to studies that assessed AP; nonetheless, BP [144, 145] showed both safety and feasibility in in- and outpatients. The device has been also assessed in a randomized crossover trial conducted at home for 11 days and allowed a reduction in mean glucose levels and in time spent in hypo [146]. Notably, there were no physical activity limitations and the patients didn’t have to input the correct amount of CHO at each meal but just the meal size. A comparison between AP and BP was performed by Haidar, who showed an improvement of glucose control during BP use in an overnight period in both children and adolescents, with less strong evidence in real life in adults [147]. BP has some limitations related to the necessity of wear 2 insulin pumps and a lack of evidence of long-term effects of the continuous administration of subcutaneous glucagon. To minimize the impact of wearing 2 different pumps, a single wearable device integrating all components into one single device much more manageable in the real-life has been developed [148]. As shown in Table 3, some meta-analyses evaluated the efficacy of CLC in the outpatient setting, comparing different AP models with standard therapy (SAP). For example, Weisman in the first meta-analyses about AP efficacy reported that time in the target was 12·59% higher with artificial pancreas systems (p < 0·0001), and BP was associated with a greater improvement in time in the target and a reduction of time spent in hypoglycemia. [102]. A second meta-analysis performed by Bekiari and colleagues in 2018 confirmed these data[101], both overnight and over 24 h, and AP efficacy was confirmed even considering the pediatric population, separately [103].
4 Biological approach
The definitive cure of diabetes may probably come from the biological approaches since they aim to replace the secretion of insulin indefinitely.
4.1 State of art
The treatment of diabetes with exogenous insulin is often problematic due to recurrent hyper-and hypoglycemic episodes. In selected patients requiring a kidney transplant or suffering from recurrent severe hypoglycemia despite optimal medical therapy, pancreas or isolated islet transplantation can restore normal glucose metabolism.
4.1.1 Pancreas transplantation
Pancreas transplantation (PT) is an option for selected subjects: however, it requires major abdominal surgery. PT demonstrated its efficacy in restore normoglycemia, stabilize complications, and reduce the burden of hypoglycemia [149]. This approach is often contemplated in subjects who required previous/simultaneous kidney transplant for end-stage kidney disease since these subjects already require major surgery and immunosuppression: this strategy accounts for the majority of PT. New immunosuppressant agents have improved organ survival with a 5 years organ survival rate between 55 and 70%. The survival rate is increased when the pancreas is transplanted simultaneously to the kidney [150]. However, surgical intervention and immunosuppression effects, limit this option to a relatively small number of subjects.
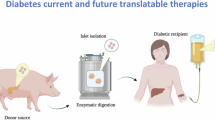
4.1.2 Islet transplantation
Islet Transplantation (IT) has been introduced 20 years ago: this procedure is more acceptable by T1Ds since it is less invasive and repeatable and could be proposed to patients who are ineligible for PT. Islets are isolated from donor pancreases, purified, injected into the portal vein to obtain their engraftment in the liver [151]. When compared to optimal insulin therapy, IT demonstrated higher efficacy in reducing severe hypoglycemia [152] and preventing microvascular complications [153]. In medium long-term efficacy, IT is lower than that of PT in providing insulin independence with approximately 50% of patients remained insulin-independent at 5 years [154]. Similar to PT, these approaches are limited by the number of donor organs and by the need for immunosuppression. Encapsulation of islet has been evaluated, to prevent rejection and immune response. The presence of encapsulation creates a barrier that prevents the access of immune cells, thus limiting the necessity of immunosuppression, but also precluding optimal vascularization. Results were not satisfactory in terms of c peptide production and metabolic control but lead to a new strategy to protect transplanted cells [155].
5 Future perspectives
5.1 Beta cells replacement
PT and IT limits have sparked research for alternative sources of beta cells, potentially unlimited, and without the need for immunosuppression. Xenotransplantation represents a possible solution to the donor shortage and recent research in genetic modification and immunosuppressive regimens have increased interest in this area. Until now small clinical studies have considered this possibility, especially using pigs beta cells. Major barriers to xenotransplantation are represented by an instant blood-mediated inflammatory reaction, chronic rejection, and the risk of transmission of porcine infectious diseases. To overcome the risk of acute rejection, related to different cell surface epitopes between humans and donor, genetically modified Xeno islet have been created, using gene-editing techniques to alter proteins in cells surface. As demonstrated for human beta cell transplantation, encapsulation could protect also Xeno islet from immune attack. There are persistent barriers to xenotransplantation and further data are necessary to establish an ideal genetically modified porcine islet to evaluate the possibility of clinical studies [156].
Future strategies for beta-cell replacements are based on stem cell (SC) to create insulin-producing cells (IPC) from SC [157,158,159,160,161]. The first problem to solve is the source of IPC: these cells could be produced from a stem cell-derived from human embryonic cells (EC) [162] or human-induced pluripotent stem cells (IPSC) [163]. EC are pluripotent cells derived from the blastocyst that can proliferate indefinitely, and differentiate in different tissues. At variance, IPSC is derived from adult mature tissue, and is re-programmed by appropriate stimuli to pluripotent cells [164]. These types of stem cells are similar in pluripotent capacity, and have the potential to create IPC: however, there are significant ethical issues in using EC so that the main source of stem cells can be considered the IPSC. Pivotal studies in this field reported insulin-producing cells obtained either by EC or IPCS but their generation rate was low and with a poor secretory response to high glucose, probably due to the low differentiation efficiency of protocols employed [165,166,167]. In 2014 a detailed protocol to generate mature and functional insulin-producing cells from SC was published, describing 7 sequential stages to obtain beta cells able to reverse hyperglycemia in diabetic mice [164]. The 7 stages were defined by endoderm, primitive gut hub, posterior foregut, pancreatic endoderm, pancreatic endocrine precursors, immature beta cells, and maturing beta cells. Veres demonstrated that during these processes only 45% of produced cells are beta-cell [168] since, at each step of the process, a consistent fraction of the cells deviated from the desired path: the consequence of this was the generation of an array of different cell phenotypes such as alfa cells, non-endocrine pancreatic exocrine cells, enterochromaffin cells, and also replicating cells which poses a serious question about malignancy risk. The beta cells demonstrated their functionality when transplanted in diabetic mice in 40 days, showing secretion of C-peptide and insulin in response to glucose. Maturation of the beta cells could be obtained in vitro with the administration of different small-molecules and hormones, or in vivo, with the transplantation of pancreatic progenitor [169]: it has been established that, in vivo, maturation is not related to the pancreas environment since maturation was obtained also after pancreas progenitor transplantation in mice kidney surface [170], thus suggesting that a critical point is a micro vascularization that supplies nutrients and oxygen. In this context, several groups are developing encapsulation devices that allow both substrate supply for beta cells and protection against immune attack; furthermore, encapsulation has the potential to limit the risk of neoplasm formation due to the presence of undifferentiated cells. The preparation of the ideal device should contemplate the biocompatibility of the membrane, the possibility of exposure to blood to allow adequate metabolism for the cells, the adequate release of insulin, and sufficient isolation from immune-competent cells. Thus, major difficulties are related to the finding of an optimal balance between permeability and defense against the host's immune response. Novel cell encapsulation systems are being developed to overcome these problems, and studies in humans are ongoing to evaluate the role of this approach [171]. A different strategy consists of the production of IPC directly from diabetic patients to overcome several obstacles related to the immune response. It has been documented that the production of IPC from skin fibroblasts of T1Ds is reliable [172], and it has been confirmed that these cells are similar to adult beta cells and able to produce insulin in response to glucose variation both in vitro and in murine models [173]. Limits of this approach are related to differences intrinsic to patients with T1Ds, with the need to develop different stem cell lines. In conclusion, today the main challenges in developing a beta cell replacement using stem cells are related to 1 the efficient generation of safe and functional insulin-producing cells (pancreatic progenitor or beta cells); 2. the transplantation of cells that do not spark the immune response; 3. conditions that allow adequate nutritional support; 4. the protection from the risk of malignant transformation; 5. a durable normalization of glycemia. However, several progress has been performed in the last decades suggesting that stem cell-based therapy for T1DM could represent the most advanced approach for a definitive cure of T1D.
5.2 Gene therapy
Gene therapy has also been considered to achieve permanent restoration of insulin production [174]: studies in this field confirmed the possibility of obtaining ectopic insulin production from different cells, for example, keratinocytes or fibroblasts [175, 176] using ex vivo gene transfer methods. Using in vitro techniques, gene transfer genetically modified cells in vitro, then they are transplanted into the subjects: in animal models, this approach allowed a secretion of insulin able to promote glucose uptake and normalize glycemia. In vivo gene transfer is performed by viral vectors that modify cells, such hepatocytes, to produce insulin: in murine models, glucose-dependent insulin production by the liver has been demonstrated, with a parallel correction of hyperglycemia [177]. However, gene therapy has some limitations related to risk related to genes chromosomal integration, viral vector safety, and immune response against virus used in vivo transfer. Gene therapy could be also applied to other mechanisms involved in overt diabetes progression: in vivo gene transfer of antiapoptotic factors demonstrated an increased number of beta cells survival by reducing apoptosis induced by the immune response [178]. Although there are no studies available in humans, the results obtained in animal models suggest a possible role of this approach in the future.
5.3 Prevention of T1D
T1D is an autoimmune disease: with his background in mind, trials have been conducted to halt or slow down the natural history of the disease. Viruses have been considered responsible for the immune response, so vaccination against viruses associated with T1D have been tested [179]. Also, the induction of immune tolerance to beta-cell antigens, such as GAD or insulin, have been explored [180, 181]. None of these studies was successful since they did not delay beta cell destruction [182]. Similar results were obtained with subcutaneous insulin administration [183]. The gut microbiome has a role in immune regulation, and it has been shown a correlation between specific bacterial species and T1D development. Although there is no evidence about the modification in microbiota in the prevention of T1D, this hypothesis could be explored in the future to determine how the gut can modulate immune regulation [184, 185]. Immunosuppressive therapy was also assessed to maintain insulin secretion in the early phase of T1D. Some studies performed in the early 90 showed that the treatment with cyclosporine increased remission rate in new-onset diabetic subjects [186] during 2 years follow up but obvious drug toxicity restrained its use. However, these data suggested that the immunosuppression was able to preserve beta cells from the immune, encouraging studies in this field. For example, in subjects with new-onset diabetes, therapy with a low dose of anti-thymocyte reduced the decline beta-cell function ad improved HbA1c more than subjects treated with placebo [187]. Anti-CD20 monoclonal antibody demonstrated a significant reduction in c peptide decline vs placebo 1 year after drug infusion [188] and also other agents were evaluated with similar results, offering new approaches for the cure shortly [189]. Overall, these data showed that the natural history of type 1 diabetes could be modified, but further studies are necessary to evaluate the long-term effect of immunosuppressive therapy. Other agents were considered for their role in inflammation and immunomodulation [190]. Recently great interest has emerged about the role of vitamin D in the prevention of T1D high-risk subjects [191]. Preclinical studies in mice demonstrated an effect on beta-cell dysfunction and inflammation [192], supported by epidemiological data that demonstrate a correlation between hypovitaminosis D and T1D [193, 194]. Unfortunately, the evidence for this link was inconclusive and further studies are necessary to test such a hypothesis. Omega-3 polyunsaturated fatty acids (O3PUFA) anti-inflammatory role has been explored but there are little data on their effect: in animal models, dietary intervention with O3PUFA reduces inflammatory markers and the incidence of T1D [195]. Epidemiological data [196] suggest a correlation between omega-3 fatty acid intake and the risk of appearance of diabetes-specific autoantibodies. In table 4 ongoing trial regarding type 1 diabetes prevention have been reported.
6 Conclusions
Although in the recent year the management of diabetes has dramatically improved, yet the disease has a remarkable impact on subjects with diabetes (Table 5). Particularly in young patients, the burden related to the chronicity and complications calls for new solutions (Table 6). The development of the first models of AP made possible the dream of creating a system able to automatically modify insulin administration through insulin pump based on the values detected by the glucose sensor. AP led to a further improvement of glycemic control with a parallel reduction of burden related to the management of diabetes, especially hypoglycemia. Unfortunately, these systems are not yet fully automated and still require the patient's intervention, especially during the and physical activity. Although the technology can be considered today the most advanced way to manage diabetes, a definitive cure could be obtained only through the biological approaches that guarantee a constant replacement of insulin such as pancreas transplants, and islet cell transplants. In perspective, stem cells, and the possibility of creating new potentially, unlimited beta cells, likely not requiring immunosuppressive therapy, could be finally the cure for diabetes (Fig. 2).
References
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993 Sep 30;329(14):977–86. https://doi.org/10.1056/NEJM199309303291401. PMID: 8366922.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000 Feb 10;342(6):381–9. https://doi.org/10.1056/NEJM200002103420603. Erratum in: N Engl J Med 2000 May 4;342(18):1376. PMID: 10666428; PMCID: PMC2630213.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–53. https://doi.org/10.1056/NEJMoa052187. PMID: 16371630; PMCID: PMC2637991.
Umpierrez G, Korytkowski M. Diabetic emergencies—ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12:222–32.
Leese GP, Wang J, Broomhall J. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–80.
Cengiz E, Xing D, Wong JC, et al. T1D Exchange Clinic Network. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange Clinic Registry. Pediatr Diabetes 2013; 14: 447–54.
Weinstock RS, Xing D, Maahs DM, et al. T1D Exchange Clinic Network. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2013; 98: 3411–19.
Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–12.
Urakami T. Severe Hypoglycemia: Is It Still a Threat for Children and Adolescents With Type 1 Diabetes? Front Endocrinol (Lausanne). 2020;15(11):609. https://doi.org/10.3389/fendo.2020.00609.PMID:33042005;PMCID:PMC7523511.
Rossi MC, Nicolucci A, Ozzello A, Gentile S, Aglialoro A, Chiambretti A, Baccetti F, Gentile FM, Romeo F, Lucisano G, Giorda CB; HYPOS-1 Study Group of AMD. Impact of severe and symptomatic hypoglycemia on quality of life and fear of hypoglycemia in type 1 and type 2 diabetes. Results of the Hypos-1 observational study. Nutr Metab Cardiovasc Dis. 2019 Jul;29(7):736–743. https://doi.org/10.1016/j.numecd.2019.04.009. Epub 2019 May 6. PMID: 31153746.
Zhang Y, Li S, Zou Y, Wu X, Bi Y, Zhang L, Yuan Y, Gong W, Hayter M. Fear of hypoglycemia in patients with type 1 and 2 diabetes: a systematic review. J Clin Nurs. 2020 Oct 22. https://doi.org/10.1111/jocn.15538. Epub ahead of print. PMID: 33091198.
Bronner MB, Peeters MAC, Sattoe JNT, van Staa A. The impact of type 1 diabetes on young adults’ health-related quality of life. Health Qual Life Outcomes. 2020;18(1):137. https://doi.org/10.1186/s12955-020-01370-8.PMID:32398086;PMCID:PMC7218580.
Totka JP, Snethen JA, Cox ED. Youth and Parent Health-Related Quality of Life and Association With Glycemic Outcomes in Preadolescents and Adolescents With Type 1 Diabetes. J Pediatr Health Care. 2020 Sep 11:S0891–5245(20)30208-X. https://doi.org/10.1016/j.pedhc.2020.07.015. Epub ahead of print. PMID: 32928601
Smith-Palmer J, Bae JP, Boye KS, Norrbacka K, Hunt B, Valentine WJ. Evaluating health-related quality of life in type 1 diabetes: a systematic literature review of utilities for adults with type 1 diabetes. Clinicoecon Outcomes Res. 2016;7(8):559–71. https://doi.org/10.2147/CEOR.S114699.PMID:27785079;PMCID:PMC5063604.
Janež A, Guja C, Mitrakou A, Lalic N, Tankova T, Czupryniak L, Tabák AG, Prazny M, Martinka E, Smircic-Duvnjak L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: a Narrative Review. Diabetes Ther. 2020 Feb;11(2):387–409. https://doi.org/10.1007/s13300-019-00743-7. Epub 2020 Jan 4. PMID: 31902063; PMCID: PMC6995794.
Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL−1. Diabetes Care. 2015;38:637–43.
Heise T, Hovelmann U, Nosek L, Hermanski L, Bottcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol. 2015;11:1193–201.
Heller S, Mathieu C, Kapur R, Wolden ML, Zinman B. A meta-analysis of rate ratios for nocturnal confirmed hypoglycaemia with insulin degludec vs. insulin glargine using different definitions for hypoglycaemia. Diabet Med. 2016 Apr;33(4):478–87. https://doi.org/10.1111/dme.13002.
Vargas-Uricoechea H. Efficacy and Safety of Insulin Glargine 300 U/mL versus 100 U/mL in Diabetes Mellitus: A Comprehensive Review of the Literature. J Diabetes Res. 2018;12(2018):2052101. https://doi.org/10.1155/2018/2052101.
Norgaard K, Sukumar N, Rafnsson SB, Saravanan P. Efficacy and safety of rapid-acting insulin analogs in special populations with type 1 diabetes or gestational diabetes: systematic review and meta-analysis. Diabetes Ther. 2018;9:891–917.
Pal R, Banerjee M, Bhadada SK. Glycemic Efficacy And Safety Of Mealtime Faster-Acting Insulin Aspart Administered By Injection As Compared To Insulin Aspart In People With Diabetes Mellitus: A Meta-Analysis Of Randomized Controlled Trials. Diabet Med. 2021;8:e14515. https://doi.org/10.1111/dme.14515.
Nimri R, Nir J, Phillip M. Insulin Pump Therapy. Am J Ther. 2020 Jan/Feb;27(1):e30-e41. doi: https://doi.org/10.1097/MJT.0000000000001097. PMID: 31833871.
Wright LA, Hirsch IB. Non-insulin treatments for Type 1 diabetes: critical appraisal of the available evidence and insight into future directions. Diabet Med. 2019;36(6):665–78. https://doi.org/10.1111/dme.13941 Epub 2019 Mar 27 PMID: 30801765.
Li N, Chen R, Liu K. Sodium-Glucose Cotransporter Inhibitors for the Treatment of Type 1 Diabetes Mellitus. Clin Drug Investig. 2020;40(11):991–1000. https://doi.org/10.1007/s40261-020-00949-9.
Egan AM, Montori VM. Review: In adults with type 1 diabetes, SGLT-2 inhibitors reduce HbA1c but increase diabetic ketoacidosis. Ann Intern Med. 2018 Jul 17;169(2):JC3. doi: https://doi.org/10.7326/ACPJC-2018-169-2-003.
Danne T, Garg S, Peters AL, Buse JB, Mathieu C, Pettus JH, Alexander CM, Battelino T, Ampudia-Blasco FJ, Bode BW, Cariou B, Close KL, Dandona P, Dutta S, Ferrannini E, Fourlanos S, Grunberger G, Heller SR, Henry RR, Kurian MJ, Kushner JA, Oron T, Parkin CG, Pieber TR, Rodbard HW, Schatz D, Skyler JS, Tamborlane WV, Yokote K, Phillip M. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care. 2019;42(6):1147–54. https://doi.org/10.2337/dc18-2316.
Meng H, Zhang A, Liang Y, Hao J, Zhang X, Lu J. Effect of metformin on glycaemic control in patients with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2018 May;34(4):e2983. doi: https://doi.org/10.1002/dmrr.2983. Epub 2018 Feb 15. PMID: 29351716.
Dimitrios P, Michael D, Vasilios K, Konstantinos S, Konstantinos I, Ioanna Z, Konstantinos P, Spyridon B, Asterios K. Liraglutide as Adjunct to Insulin Treatment in Patients with Type 1 Diabetes: A Systematic Review and Meta-analysis. Curr Diabetes Rev. 2020;16(4):313–26. https://doi.org/10.2174/1573399815666190614141918 (PMID: 31203802).
Galindo RJ, Aleppo G. Continuous Glucose Monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020 Oct 13:108502. https://doi.org/10.1016/j.diabres.2020.108502. Epub ahead of print. PMID: 33065179.
Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(3):198–206. https://doi.org/10.1016/S2213-8587(14)70248-7 (Epub 2015 Feb 6 PMID: 25660575).
Vergès B. Cardiovascular disease in type 1 diabetes: A review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020 Sep 28:S1262–3636(20)30126–9. https://doi.org/10.1016/j.diabet.2020.09.001. Epub ahead of print. PMID: 32998054.
Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2017;55(1):77–84. https://doi.org/10.1007/s12020-016-1039-x (Epub 2016 Aug 1 PMID: 27477293).
Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765–74. https://doi.org/10.1111/j.1464-5491.2008.02486.x (PMID: 18644063).
Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol. 2010;47(Suppl 1):77–81. https://doi.org/10.1007/s00592-009-0132-5 (Epub 2009 Jun 6 PMID: 19504039).
Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials. Acta Diabetol. 2019;56(9):973–80. https://doi.org/10.1007/s00592-019-01326-5 (Epub 2019 Apr 3 PMID: 30945047).
Qin Y, Yang LH, Huang XL, Chen XH, Yao H. Efficacy and Safety of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Injections on Type 1 Diabetes Children: A Meta-Analysis of Randomized Control Trials. J Clin Res Pediatr Endocrinol. 2018 Nov 29;10(4):316–323. https://doi.org/10.4274/jcrpe.0053. Epub 2018 Jul 17. PMID: 30015622; PMCID: PMC6280319
Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, Siebenhofer A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941–51. https://doi.org/10.1007/s00125-008-0974-3 (Epub 2008 Mar 20 PMID: 18351320).
Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD005103. https://doi.org/10.1002/14651858.CD005103.pub2. PMID: 20091571.
Müller-Godeffroy E, Treichel S, Wagner VM. German Working Group for Paediatric Pump Therapy. Investigation of quality of life and family burden issues during insulin pump therapy in children with Type 1 diabetes mellitus--a large-scale multicentre pilot study. Diabet Med. 2009 May;26(5):493–501. https://doi.org/10.1111/j.1464-5491.2009.02707.x. PMID: 19646189.
EQuality1 Study Group--Evaluation of QUALITY of Life and Costs in Diabetes Type 1, Nicolucci A, Maione A, Franciosi M, Amoretti R, Busetto E, Capani F, Bruttomesso D, Di Bartolo P, Girelli A, Leonetti F, Morviducci L, Ponzi P, Vitacolonna E. Quality of life and treatment satisfaction in adults with Type 1 diabetes: a comparison between continuous subcutaneous insulin infusion and multiple daily injections. Diabet Med. 2008 Feb;25(2):213–20. https://doi.org/10.1111/j.1464-5491.2007.02346.x. Epub 2008 Jan 14. PMID: 18201210.
Realsen J, Goettle H, Chase HP. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol Ther. 2012;14(12):1149–54. https://doi.org/10.1089/dia.2012.0161 (Epub 2012 Sep 25 PMID: 23009106).
Rosenlund S, Hansen TW, Andersen S, Rossing P. Effect of 4 years subcutaneous insulin infusion treatment on albuminuria, kidney function and HbA1c compared with multiple daily injections: a longitudinal follow-up study. Diabet Med. 2015;32(11):1445–52. https://doi.org/10.1111/dme.12950 (Epub 2015 Oct 6 PMID: 26331364).
Steineck I, Cederholm J, Eliasson B, Rawshani A, Eeg-Olofsson K, Svensson AM, Zethelius B, Avdic T, Landin-Olsson M, Jendle J, Gudbjörnsdóttir S; Swedish National Diabetes Register. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015 Jun 22;350:h3234. https://doi.org/10.1136/bmj.h3234. PMID: 26100640; PMCID: PMC4476263.
Spaan N, Teplova A, Stam G, Spaan J, Lucas C. Systematic review: continuous intraperitoneal insulin infusion with implantable insulin pumps for diabetes mellitus. Acta Diabetol. 2014;51(3):339–51. https://doi.org/10.1007/s00592-014-0557-3.
van Dijk PR, Logtenberg SJ, Groenier KH, Gans RO, Kleefstra N, Bilo HJ. Continuous intraperitoneal insulin infusion in type 1 diabetes: a 6-year post-trial follow-up. BMC Endocr Disord. 2014;7(14):30. https://doi.org/10.1186/1472-6823-14-30.
Haardt MJ, Selam JL, Slama G, Bethoux JP, Dorange C, Mace B, Ramaniche ML, Bruzzo F. A cost-benefit comparison of intensive diabetes management with implantable pumps versus multiple subcutaneous injections in patients with type I diabetes. Diabetes Care. 1994;17(8):847–51. https://doi.org/10.2337/diacare.17.8.847.
Facchinetti A. Continuous Glucose Monitoring Sensors: Past, Present and Future Algorithmic Challenges. Sensors (Basel). 2016 Dec 9;16(12). https://doi.org/10.3390/s16122093.
Rodbard D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol Ther. 2016;18(Suppl 2):S3–13. https://doi.org/10.1089/dia.2015.0417.
Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018 Apr-Jun;12(2):181–187. https://doi.org/10.1016/j.dsx.2017.09.005. Epub 2017 Sep 22. PMID: 28967612.
Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;7(343):d3805. https://doi.org/10.1136/bmj.d3805.PMID:21737469;PMCID:PMC3131116.
Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, Schierloh U, Sulli N, Bolinder J. SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012 Dec;55(12):3155–62. https://doi.org/10.1007/s00125-012-2708-9. Epub 2012 Sep 11. PMID: 22965294; PMCID: PMC3483098.
Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, Kollman C, Kruger D, McGill JB, Polonsky W, Toschi E, Wolpert H, Price D. DIAMOND Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017 Jan 24;317(4):371–378. https://doi.org/10.1001/jama.2016.19975. PMID: 28118453.
Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, Schwarz E, Ólafsdóttir AF, Frid A, Wedel H, Ahlén E, Nyström T, Hellman J. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA. 2017;317(4):379–87. https://doi.org/10.1001/jama.2016.19976.Erratum.In:JAMA.2017May9;317(18):1912 (PMID: 28118454).
Golicki DT, Golicka D, Groele L, Pankowska E. Continuous Glucose Monitoring System in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2008;51(2):233–40. https://doi.org/10.1007/s00125-007-0884-9 (Epub 2007 Dec 1 PMID: 18060380).
Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger-stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in Type I diabetic patients: a systematic review. Diabetes Res Clin Pract. 2008;81(1):79–87. https://doi.org/10.1016/j.diabres.2008.02.014 (Epub 2008 Apr 15 PMID: 18417243).
Szypowska A, Ramotowska A, Dzygalo K, Golicki D. Beneficial effect of real-time continuous glucose monitoring system on glycemic control in type 1 diabetic patients: systematic review and meta-analysis of randomized trials. Eur J Endocrinol. 2012;166(4):567–74. https://doi.org/10.1530/EJE-11-0642 (Epub 2011 Nov 17 PMID: 22096111).
Floyd B, Chandra P, Hall S, Phillips C, Alema-Mensah E, Strayhorn G, Ofili EO, Umpierrez GE. Comparative analysis of the efficacy of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes mellitus. J Diabetes Sci Technol. 2012;6(5):1094–102. https://doi.org/10.1177/193229681200600513.PMID:23063035;PMCID:PMC3570843.
Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, Wilson LM, Haberl EB, Brick J, Bass EB, Golden SH. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–47. https://doi.org/10.7326/0003-4819-157-5-201209040-00508 (PMID: 22777524).
Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD008101. https://doi.org/10.1002/14651858.CD008101.pub2. PMID: 22258980; PMCID: PMC6486112.
Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;23(5):39. https://doi.org/10.1186/1758-5996-5-39.PMID:23876067;PMCID:PMC3728077.
Benkhadra K, Alahdab F, Tamhane S, Wang Z, Prokop LJ, Hirsch IB, Raccah D, Riveline JP, Kordonouri O, Murad MH. Real-time continuous glucose monitoring in type 1 diabetes: a systematic review and individual patient data meta-analysis. Clin Endocrinol (Oxf). 2017;86(3):354–60. https://doi.org/10.1111/cen.13290 (Epub 2017 Jan 16 PMID: 27978595).
Wong JC, Foster NC, Maahs DM, Raghinaru D, Bergenstal RM, Ahmann AJ, Peters AL, Bode BW, Aleppo G, Hirsch IB, Kleis L, Chase HP, DuBose SN, Miller KM, Beck RW, Adi S; T1D Exchange Clinic Network. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care. 2014 Oct;37(10):2702–9. https://doi.org/10.2337/dc14-0303. Epub 2014 Jul 10. PMID: 25011947; PMCID: PMC4392936.
Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015;17(3):177–86. https://doi.org/10.1089/dia.2014.0272.
Thabit H, Prabhu JN, Mubita W, Fullwood C, Azmi S, Urwin A, Doughty I, Leelarathna L. Use of Factory-Calibrated Real-time Continuous Glucose Monitoring Improves Time in Target and HbA1c in a Multiethnic Cohort of Adolescents and Young Adults With Type 1 Diabetes: The MILLENNIALS Study. Diabetes Care. 2020;43(10):2537–43. https://doi.org/10.2337/dc20-0736 (Epub 2020 Jul 28 PMID: 32723843).
Kropff J, Choudhary P, Neupane S, Barnard K, Bain SC, Kapitza C, Forst T, Link M, Dehennis A, DeVries JH. Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter. Pivotal Trial Diabetes Care. 2017;40(1):63–8. https://doi.org/10.2337/dc16-1525 (Epub 2016 Nov 4 PMID: 27815290).
Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, Denham DS, Atiee G, Bode BW, Walters SJ, Kelley L, Bailey TS. A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II. Diabetes Technol Ther. 2018 Mar;20(3):197–206. https://doi.org/10.1089/dia.2017.0142. Epub 2018 Jan 30. PMID: 29381090; PMCID: PMC5867508.
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–63. https://doi.org/10.1016/S0140-6736(16)31535-5 (Epub 2016 Sep 12 PMID: 27634581).
Tyndall V, Stimson RH, Zammitt NN, Ritchie SA, McKnight JA, Dover AR, Gibb FW. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia. 2019 Aug;62(8):1349–1356. https://doi.org/10.1007/s00125-019-4894-1. Epub 2019 Jun 9. PMID: 31177314; PMCID: PMC6647076.
Gordon I, Rutherford C, Makarounas-Kirchmann K, Kirchmann M. Meta-analysis of average change in laboratory-measured HbA1c among people with type 1 diabetes mellitus using the 14 day Flash Glucose Monitoring System. Diabetes Res Clin Pract. 2020;164:108158. https://doi.org/10.1016/j.diabres.2020.108158 (Epub 2020 Apr 23 PMID: 32333970).
Al Hayek AA, Al Dawish MA. Assessing Diabetes Distress and Sleep Quality in Young Adults with Type 1 Diabetes Using FreeStyle Libre: A Prospective Cohort Study. Diabetes Ther. 2020 Jul;11(7):1551–1562. https://doi.org/10.1007/s13300-020-00849-3. Epub 2020 Jun 4. PMID: 32495021; PMCID: PMC7324459.
Polonsky WH. Psychosocial Aspects of Diabetes Technology: Adult Perspective. Endocrinol Metab Clin North Am. 2020;49(1):143–55. https://doi.org/10.1016/j.ecl.2019.10.003 (Epub 2019 Dec 9 PMID: 31980114).
Diabetes Research In Children Network (DirecNet) Study Group, Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, Tsalikian E, Tamborlane W, Wysocki T, Ruedy K, Beck R. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator). Pediatr Diabetes. 2008 Apr;9(2):142–7. https://doi.org/10.1111/j.1399-5448.2007.00301.x. Epub 2008 Jan 24. PMID: 18221427; PMCID: PMC2390770.
Klonoff DC, Kerr D. A Simplified Approach Using Rate of Change Arrows to Adjust Insulin With Real-Time Continuous Glucose Monitoring. J Diabetes Sci Technol. 2017 Nov;11(6):1063–1069. https://doi.org/10.1177/1932296817723260. Epub 2017 Sep 8. PMID: 28884599; PMCID: PMC5951054.
Laffel LM, Aleppo G, Buckingham BA, Forlenza GP, Rasbach LE, Tsalikian E, Weinzimer SA, Harris DR. A Practical Approach to Using Trend Arrows on the Dexcom G5 CGM System to Manage Children and Adolescents With Diabetes. J Endocr Soc. 2017;1(12):1461–76. https://doi.org/10.1210/js.2017-00389.PMID:29344578;PMCID:PMC5760209.
Ziegler R, von Sengbusch S, Kröger J, Schubert O, Werkmeister P, Deiss D, Siegmund T. Therapy Adjustments Based on Trend Arrows Using Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2019 Jul;13(4):763–773. https://doi.org/10.1177/1932296818822539. Epub 2019 Jan 22. PMID: 30666883; PMCID: PMC6610609
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ 3rd, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Nørgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer SA, Phillip M. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019 Aug;42(8):1593–1603. https://doi.org/10.2337/dci19-0028. Epub 2019 Jun 8. PMID: 31177185; PMCID: PMC6973648.
Heinemann L, Freckmann G, Müller-Wieland D, Kellerer M. Critical Reappraisal of the Time-in-Range: Alternative or Useful Addition to Glycated Hemoglobin? J Diabetes Sci Technol. 2020 Sep;14(5):922–927. https://doi.org/10.1177/1932296819883885. Epub 2019 Nov 1. PMID: 31675907; PMCID: PMC7753853
Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Nørgaard K. Improved Time in Range Over 1 Year Is Associated With Reduced Albuminuria in Individuals With Sensor-Augmented Insulin Pump-Treated Type 1 Diabetes. Diabetes Care. 2020;43(11):2882–5. https://doi.org/10.2337/dc20-0909 (Epub 2020 Sep 4 PMID: 32887707).
Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care. 2018;41(11):2370–6. https://doi.org/10.2337/dc18-1131 (Epub 2018 Sep 10 PMID: 30201847).
Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, Mo Y, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W, Zhou J. Time in Range Is Associated with Carotid Intima-Media Thickness in Type 2 Diabetes. Diabetes Technol Ther. 2020;22(2):72–8. https://doi.org/10.1089/dia.2019.0251 (Epub 2019 Oct 11 PMID: 31524497).
Weinstock RS, Aleppo G, Bailey TS, Bergenstal RM, Fisher WA, Greenwood DA, Young LA. The Role of Blood Glucose Monitoring in Diabetes Management. Arlington (VA): American Diabetes Association; 2020.
Engler R, Routh TL, Lucisano JY. Adoption Barriers for Continuous Glucose Monitoring and Their Potential Reduction With a Fully Implanted System: Results From Patient Preference Surveys. Clin Diabetes. 2018;36(1):50–8. https://doi.org/10.2337/cd17-0053.PMID:29382979;PMCID:PMC5774999.
Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R; DPV-Wiss-Initiative. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011 Feb;12(1):11–7. https://doi.org/10.1111/j.1399-5448.2010.00650.x. PMID: 20337978.
Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington (VA): American Diabetes Association; 2018 Aug. PMID: 30958664.
Freckmann G, Baumstark A, Jendrike N, Mende J, Schauer S, Link M, Pleus S, Haug C. Impact of Two Different Reference Measurement Procedures on Apparent System Accuracy of 18 CE-Marked Current-Generation Blood Glucose Monitoring Systems. J Diabetes Sci Technol. 2020 Aug 19:1932296820948873. https://doi.org/10.1177/1932296820948873. Epub ahead of print. PMID: 32814455.
Clements MA, Staggs VS. A Mobile App for Synchronizing Glucometer Data: Impact on Adherence and Glycemic Control Among Youths With Type 1 Diabetes in Routine Care. J Diabetes Sci Technol. 2017 May;11(3):461–467. https://doi.org/10.1177/1932296817691302. Epub 2017 Feb 9. PMID: 28745097; PMCID: PMC5505434
Vallejo-Mora MD, Carreira-Soler M, Linares-Parrado F, Olveira G, Rojo-Martínez G, Domínguez-López M, Ruiz-de-Adana-Navas MS, González-Romero MS. The Calculating Boluses on Multiple Daily Injections (CBMDI) study: A randomized controlled trial on the effect on metabolic control of adding a bolus calculator to multiple daily injections in people with type 1 diabetes. J Diabetes. 2017;9(1):24–33. https://doi.org/10.1111/1753-0407.12382 (Epub 2016 Apr 13 PMID: 26848934).
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Wilson DM, Xing D, Beck RW, Block J, Bode B, Fox LA, Hirsch I, Kollman C, Laffel L, Ruedy KJ, Steffes M, Tamborlane WV. Hemoglobin A1c and mean glucose in patients with type 1 diabetes: analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care. 2011 Mar;34(3):540–4. https://doi.org/10.2337/dc10-1054. Epub 2011 Jan 25. PMID: 21266647; PMCID: PMC3041177.
Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Ahmann AJ, Welsh JB, Lee SW, Kaufman FR; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013 Jul 18;369(3):224–32. https://doi.org/10.1056/NEJMoa1303576. Epub 2013 Jun 22. PMID: 23789889.
Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, Shaw JA, Pickup JC, Amiel SA. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34(9):2023–5. https://doi.org/10.2337/dc10-2411.PMID:21868778;PMCID:PMC3161284.
Buckingham BA, Raghinaru D, Cameron F, Bequette BW, Chase HP, Maahs DM, Slover R, Wadwa RP, Wilson DM, Ly T, Aye T, Hramiak I, Clarson C, Stein R, Gallego PH, Lum J, Sibayan J, Kollman C, Beck RW; In Home Closed Loop Study Group. Predictive Low-Glucose Insulin Suspension Reduces Duration of Nocturnal Hypoglycemia in Children Without Increasing Ketosis. Diabetes Care. 2015 Jul;38(7):1197–204. https://doi.org/10.2337/dc14-3053. Epub 2015 Jun 6. Erratum in: Diabetes Care. 2015 Sep;38(9):1813. PMID: 26049549; PMCID: PMC4477332.
Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of Hypoglycemia With Predictive Low Glucose Insulin Suspension in Children With Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2017;40(6):764–70. https://doi.org/10.2337/dc16-2584 (Epub 2017 Mar 28 PMID: 28351897).
Alotaibi A, Al Khalifah R, McAssey K. The efficacy and safety of insulin pump therapy with predictive low glucose suspend feature in decreasing hypoglycemia in children with type 1 diabetes mellitus: A systematic review and meta-analysis. Pediatr Diabetes. 2020 Aug 1. https://doi.org/10.1111/pedi.13088. Epub ahead of print. PMID: 32738022.
Tubili C, Pollakova D, Nardone MR, Di Folco U. Predictive Low Glucose Suspend Algorithm in Real Life: A Five-Year Follow-Up Retrospective Analysis. J Diabetes Sci Technol. 2020 Aug 29:1932296820952107. https://doi.org/10.1177/1932296820952107. Epub ahead of print. PMID: 32865016.
Beato-Víbora PI, Quirós-López C, Lázaro-Martín L, Martín-Frías M, Barrio-Castellanos R, Gil-Poch E, Arroyo-Díez FJ, Giménez-Álvarez M. Impact of Sensor-Augmented Pump Therapy with Predictive Low-Glucose Suspend Function on Glycemic Control and Patient Satisfaction in Adults and Children with Type 1 Diabetes. Diabetes Technol Ther. 2018;20(11):738–43. https://doi.org/10.1089/dia.2018.0199 (Epub 2018 Sep 25 PMID: 30256132).
Pinsker JE, Leas S, Müller L, Habif S. Real world improvements in hypoglycemia in an insulin-dependent cohort with diabetes mellitus pre/post tandem basal-iq technology remote software update. Endocr Pract. 2020 Mar 11. https://doi.org/10.4158/EP-2019-0554. Epub ahead of print. PMID: 32160040.
Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672–82. https://doi.org/10.2337/db11-0654.PMID:22025773;PMCID:PMC3198099.
Fuchs J, Hovorka R. Closed-loop control in insulin pumps for type-1 diabetes mellitus: safety and efficacy. Expert Rev Med Devices. 2020 Jul;17(7):707–720. https://doi.org/10.1080/17434440.2020.1784724. Epub 2020 Jul 3. PMID: 32569476; PMCID: PMC7441745.
Kumareswaran K, Elleri D, Allen JM, Harris J, Xing D, Kollman C, Nodale M, Murphy HR, Amiel SA, Heller SR, Wilinska ME, Acerini CL, Evans ML, Dunger DB, Hovorka R. Meta-analysis of overnight closed-loop randomized studies in children and adults with type 1 diabetes: the Cambridge cohort. J Diabetes Sci Technol. 2011;5(6):1352–62. https://doi.org/10.1177/193229681100500606.PMID:22226252;PMCID:PMC3262701.
Battelino T, Omladič JŠ, Phillip M. Closed loop insulin delivery in diabetes. Best Pract Res Clin Endocrinol Metab. 2015;29(3):315–25. https://doi.org/10.1016/j.beem.2015.03.001 (Epub 2015 Mar 10 PMID: 26051293).
Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich AB, Hovorka R, Tsapas A. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;18(361):k1310. https://doi.org/10.1136/bmj.k1310.PMID:29669716;PMCID:PMC5902803.
Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–12. https://doi.org/10.1016/S2213-8587(17)30167-5 (Epub 2017 May 19 PMID: 28533136).
Karageorgiou V, Papaioannou TG, Bellos I, Alexandraki K, Tentolouris N, Stefanadis C, Chrousos GP, Tousoulis D. Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis. Metabolism. 2019;90:20–30. https://doi.org/10.1016/j.metabol.2018.10.002 (Epub 2018 Oct 12 PMID: 30321535).
Farrington C, Stewart Z, Hovorka R, Murphy H. Women's Experiences of Day-and-Night Closed-Loop Insulin Delivery During Type 1 Diabetes Pregnancy. J Diabetes Sci Technol. 2018 Nov;12(6):1125–1131. https://doi.org/10.1177/1932296818800065. Epub 2018 Oct 5. PMID: 30288999; PMCID: PMC6232744.
Stewart ZA, Wilinska ME, Hartnell S, O’Neil LK, Rayman G, Scott EM, Barnard K, Farrington C, Hovorka R, Murphy HR. Day-and-Night Closed-Loop Insulin Delivery in a Broad Population of Pregnant Women With Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care. 2018;41(7):1391–9. https://doi.org/10.2337/dc17-2534 (Epub 2018 Mar 13 PMID: 29535135).
Stewart ZA, Wilinska ME, Hartnell S, Temple RC, Rayman G, Stanley KP, Simmons D, Law GR, Scott EM, Hovorka R, Murphy HR. Closed-Loop Insulin Delivery during Pregnancy in Women with Type 1 Diabetes. N Engl J Med. 2016;375(7):644–54. https://doi.org/10.1056/NEJMoa1602494 (PMID: 27532830).
Weaver KW, Hirsch IB. The Hybrid Closed-Loop System: Evolution and Practical Applications. Diabetes Technol Ther. 2018;20(S2):S216–23. https://doi.org/10.1089/dia.2018.0091 (Epub 2018 Jun 6 PMID: 29873517ss).
Stone JY, Haviland N, Bailey TS. Review of a commercially available hybrid closed-loop insulin-delivery system in the treatment of Type 1 diabetes. Ther Deliv. 2018;9(2):77–87. https://doi.org/10.4155/tde-2017-0099 (Epub 2017 Dec 13 PMID: 29235423).
Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, Brazg RL, Ilany J, Slover RH, Anderson SM, Bergenstal RM, Grosman B, Roy A, Cordero TL, Shin J, Lee SW, Kaufman FR. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017 Mar;19(3):155–163. https://doi.org/10.1089/dia.2016.0421. Epub 2017 Jan 30. PMID: 28134564; PMCID: PMC5359676.
Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, Wood MA, Buckingham BA, Kaiserman KB, Shin J, Huang S, Lee SW, Kaufman FR. Safety Evaluation of the MiniMed 670G System in Children 7–13 Years of Age with Type 1 Diabetes. Diabetes Technol Ther. 2019 Jan;21(1):11–19. https://doi.org/10.1089/dia.2018.0264. Epub 2018 Dec 26. PMID: 30585770; PMCID: PMC6350071.
Duffus SH, Ta'ani ZA, Slaughter JC, Niswender KD, Gregory JM. Increased proportion of time in hybrid closed-loop "Auto Mode" is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab. 2020 Apr;22(4):688–693. https://doi.org/10.1111/dom.13912. Epub 2019 Dec 9. PMID: 31709736; PMCID: PMC7549138.
McAuley SA, Lee MH, Paldus B, Vogrin S, de Bock MI, Abraham MB, Bach LA, Burt MG, Cohen ND, Colman PG, Davis EA, Hendrieckx C, Holmes-Walker DJ, Kaye J, Keech AC, Kumareswaran K, MacIsaac RJ, McCallum RW, Sims CM, Speight J, Stranks SN, Sundararajan V, Trawley S, Ward GM, Jenkins AJ, Jones TW, O'Neal DN. Australian JDRF Closed-Loop Research Group. Six Months of Hybrid Closed-Loop Versus Manual Insulin Delivery With Fingerprick Blood Glucose Monitoring in Adults With Type 1 Diabetes: A Randomized, Controlled Trial. Diabetes Care. 2020 Dec;43(12):3024–3033. https://doi.org/10.2337/dc20-1447. Epub 2020 Oct 14. PMID: 33055139
Stone MP, Agrawal P, Chen X, Liu M, Shin J, Cordero TL, Kaufman FR. Retrospective Analysis of 3-Month Real-World Glucose Data After the MiniMed 670G System Commercial Launch. Diabetes Technol Ther. 2018;20(10):689–92. https://doi.org/10.1089/dia.2018.0202 (Epub 2018 Aug 30 PMID: 30160523).
Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One Year Clinical Experience of the First Commercial Hybrid Closed-Loop System. Diabetes Care. 2019 Dec;42(12):2190–2196. https://doi.org/10.2337/dc19-0855. Epub 2019 Sep 23. PMID: 31548247; PMCID: PMC6868462.
Lee MH, Vogrin S, Paldus B, Jones HM, Obeyesekere V, Sims C, Wyatt SA, Ward GM, McAuley SA, MacIsaac RJ, Krishnamurthy B, Sundararajan V, Jenkins AJ, O’Neal DN. Glucose Control in Adults with Type 1 Diabetes Using a Medtronic Prototype Enhanced-Hybrid Closed-Loop System: A Feasibility Study. Diabetes Technol Ther. 2019;21(9):499–506. https://doi.org/10.1089/dia.2019.0120 (Epub 2019 Jul 2 PMID: 31264889).
Paldus B, Lee MH, Jones HM, McAuley SA, Horsburgh JC, Roem KL, Ward GM, MacIsaac RJ, Cohen N, Colman PG, Jenkins AJ, O’Neal DN. Glucose Control Using a Standard Versus an Enhanced Hybrid Closed Loop System: A Randomized Crossover Study. Diabetes Technol Ther. 2019;21(1):56–8. https://doi.org/10.1089/dia.2018.0279 (PMID: 30620641).
Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, Laffel LM, Levy CJ, Pinsker JE, Wadwa RP, Dassau E, Doyle FJ 3rd, Anderson SM, Church MM, Dadlani V, Ekhlaspour L, Forlenza GP, Isganaitis E, Lam DW, Kollman C, Beck RW; iDCL Trial Research Group. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med. 2019 Oct 31;381(18):1707–1717. https://doi.org/10.1056/NEJMoa1907863. Epub 2019 Oct 16. PMID: 31618560; PMCID: PMC7076915.
Ekhlaspour L, Forlenza GP, Chernavvsky D, Maahs DM, Wadwa RP, Deboer MD, Messer LH, Town M, Pinnata J, Kruse G, Kovatchev BP, Buckingham BA, Breton MD. Closed loop control in adolescents and children during winter sports: Use of the Tandem Control-IQ AP system. Pediatr Diabetes. 2019 Sep;20(6):759–768. https://doi.org/10.1111/pedi.12867. Epub 2019 May 23. PMID: 31099946; PMCID: PMC6679803.
Pinsker JE, Müller L, Constantin A, Leas S, Manning M, McElwee Malloy M, Singh H, Habif S. Real-World Patient Reported Outcomes and Glycemic Results with Initiation of Control-IQ Technology. Diabetes Technol Ther. 2020 Aug 26. https://doi.org/10.1089/dia.2020.0388. Epub ahead of print. PMID: 32846114.
Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, Hovorka R. Home Use of Day-and-Night Hybrid Closed-Loop Insulin Delivery in Suboptimally Controlled Adolescents With Type 1 Diabetes: A 3-Week, Free-Living, Randomized Crossover Trial. Diabetes Care. 2016 Nov;39(11):2019–2025. https://doi.org/10.2337/dc16-1094. Epub 2016 Sep 9. PMID: 27612500; PMCID: PMC5079605.
Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, Schaeffer D, Fichelle M, Schierloh U, Thiele AG, Abt D, Kojzar H, Mader JK, Slegtenhorst S, Barber N, Wilinska ME, Boughton C, Musolino G, Sibayan J, Cohen N, Kollman C, Hofer SE, Fröhlich-Reiterer E, Kapellen TM, Acerini CL, de Beaufort C, Campbell F, Rami-Merhar B, Hovorka R; KidsAP Consortium. Home Use of Day-and-Night Hybrid Closed-Loop Insulin Delivery in Very Young Children: A Multicenter, 3-Week, Randomized Trial. Diabetes Care. 2019 Apr;42(4):594–600. https://doi.org/10.2337/dc18-1881. Epub 2019 Jan 28. PMID: 30692242.
Benhamou P, Franc S, Reznik Y, Thivolet C, Schaepelynck P, Renard E, Guerci B, Chaillous L, Lukas-Croisier C, Jeandidier N, Hanaire H, Borot S, Doron M, Jallon P, Xhaard I, Melki V, Meyer L, Delemer B, Guillouche M, Schoumacker-Ley L, Farret, Raccah D, Lablanche S, Joubert M, Penfornis A, Charpentier G, on behalf of the DIABELOOP WP7 Trial Investigators. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health 2019; 1: e17–25
Hanaire H, Franc S, Borot S, Penfornis A, Benhamou PY, Schaepelynck P, Renard E, Guerci B, Jeandidier N, Simon C, Hannaert P, Xhaard I, Doron M, Huneker E, Charpentier G, Reznik Y. Efficacy of the Diabeloop closed-loop system to improve glycaemic control in patients with type 1 diabetes exposed to gastronomic dinners or to sustained physical exercise. Diabetes Obes Metab. 2020;22(3):324–34. https://doi.org/10.1111/dom.13898 (Epub 2019 Oct 16 PMID: 31621186).
Cobry EC, Berget C, Messer LH, Forlenza GP. Review of the Omnipod 5 Automated Glucose Control System Powered by Horizon for the treatment of Type 1 diabetes. Ther Deliv. 2020;11(8):507–19. https://doi.org/10.4155/tde-2020-0055 (Epub 2020 Jul 28 PMID: 32723002).
Buckingham BA, Christiansen MP, Forlenza GP, Wadwa RP, Peyser TA, Lee JB, O'Connor J, Dassau E, Huyett LM, Layne JE, Ly TT. Performance of the Omnipod Personalized Model Predictive Control Algorithm with Meal Bolus Challenges in Adults with Type 1 Diabetes. Diabetes Technol Ther. 2018 Sep;20(9):585–595. https://doi.org/10.1089/dia.2018.0138. Epub 2018 Aug 2. PMID: 30070928; PMCID: PMC6114075.
Sherr JL, Buckingham BA, Forlenza GP, Galderisi A, Ekhlaspour L, Wadwa RP, Carria L, Hsu L, Berget C, Peyser TA, Lee JB, O'Connor J, Dumais B, Huyett LM, Layne JE, Ly TT. Safety and Performance of the Omnipod Hybrid Closed-Loop System in Adults, Adolescents, and Children with Type 1 Diabetes Over 5 Days Under Free-Living Conditions. Diabetes Technol Ther. 2020 Mar;22(3):174–184. https://doi.org/10.1089/dia.2019.0286. Epub 2019 Oct 29. PMID: 31596130; PMCID: PMC7047109.
Musolino G, Dovc K, Boughton CK, Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, Schaeffer D, Fichelle M, Schierloh U, Thiele AG, Abt D, Kojzar H, Mader JK, Slegtenhorst S, Ashcroft N, Wilinska ME, Sibayan J, Cohen N, Kollman C, Hofer SE, Fröhlich-Reiterer E, Kapellen TM, Acerini CL, de Beaufort C, Campbell F, Rami-Merhar B, Hovorka R; Kidsap Consortium. Reduced burden of diabetes and improved quality of life: Experiences from unrestricted day-and-night hybrid closed-loop use in very young children with type 1 diabetes. Pediatr Diabetes. 2019 Sep;20(6):794–799. https://doi.org/10.1111/pedi.12872. Epub 2019 Jun 13. PMID: 31140654; PMCID: PMC6771658.
Kropff J, DeJong J, Del Favero S, Place J, Messori M, Coestier B, Farret A, Boscari F, Galasso S, Avogaro A, Bruttomesso D, Cobelli C, Renard E, Magni L, DeVries JH; AP@home consortium. Psychological outcomes of evening and night closed-loop insulin delivery under free living conditions in people with Type 1 diabetes: a 2-month randomized crossover trial. Diabet Med. 2017 Feb;34(2):262–271. https://doi.org/10.1111/dme.13268. Epub 2016 Oct 28. PMID: 27696520; PMCID: PMC5248649.
Jennings P, Hussain S. Do-It-Yourself Artificial Pancreas Systems: A Review of the Emerging Evidence and Insights for Healthcare Professionals. J Diabetes Sci Technol. 2020;14(5):868–77. https://doi.org/10.1177/1932296819894296 (Epub 2019 Dec 17 PMID: 31847570).
Sy SL, Munshi MM, Toschi E. Can Smart Pens Help Improve Diabetes Management? J Diabetes Sci Technol. 2020 Oct 21:1932296820965600. https://doi.org/10.1177/1932296820965600. Epub ahead of print. PMID: 33084417.
Jones MS, Goley AL, Alexander BE, Keller SB, Caldwell MM and Buse JB. Inpatient Transition to Virtual Care During COVID-19 Pandemic Diabetes Technol Ther. 2020 Jun;22(6):444–448. https://doi.org/10.1089/dia.2020.0206
Boscari F, Ferretto S, Uliana A, Avogaro A, Bruttomesso D. Efficacy of telemedicine for persons with type 1 diabetes during Covid19 lockdown. Nutr Diabetes. 2021;11(1):1. https://doi.org/10.1038/s41387-020-00147-8.PMID:33414391;PMCID:PMC7790327.
Teymourian H, Barfidokht A, Wang J. Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem Soc Rev. 2020 Oct 6. https://doi.org/10.1039/d0cs00304b. Epub ahead of print. PMID: 33020790.
Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T, Shin K, Choi SH, Hyeon T, Kim DH. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv. 2017;3(3):e1601314. https://doi.org/10.1126/sciadv.1601314.PMID:28345030;PMCID:PMC5342654.
Yao H, Shum AJ, Cowan M, Lähdesmäki I, Parviz BA. A contact lens with embedded sensor for monitoring tear glucose level. Biosens Bioelectron. 2011 Mar 15;26(7):3290–6. https://doi.org/10.1016/j.bios.2010.12.042. Epub 2010 Dec 31. PMID: 21257302; PMCID: PMC3043144.
Gupta S, Sandhu SV, Bansal H, Sharma D. Comparison of salivary and serum glucose levels in diabetic patients. J Diabetes Sci Technol. 2015 Jan;9(1):91–6. https://doi.org/10.1177/1932296814552673. Epub 2014 Oct 7. PMID: 25294888; PMCID: PMC4495535.
Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–9. https://doi.org/10.2337/dc07-1967 (Epub 2008 Feb 5 PMID: 18252903).
Forlenza GP, Cameron FM, Ly TT, Lam D, Howsmon DP, Baysal N, Kulina G, Messer L, Clinton P, Levister C, Patek SD, Levy CJ, Wadwa RP, Maahs DM, Bequette BW, Buckingham BA. Fully Closed-Loop Multiple Model Probabilistic Predictive Controller Artificial Pancreas Performance in Adolescents and Adults in a Supervised Hotel Setting. Diabetes Technol Ther. 2018 May;20(5):335–343. https://doi.org/10.1089/dia.2017.0424. Epub 2018 Apr 16. PMID: 29658779; PMCID: PMC5963546.
Dovc K, Piona C, Yeşiltepe Mutlu G, Bratina N, Jenko Bizjan B, Lepej D, Nimri R, Atlas E, Muller I, Kordonouri O, Biester T, Danne T, Phillip M, Battelino T. Faster Compared With Standard Insulin Aspart During Day-and-Night Fully Closed-Loop Insulin Therapy in Type 1 Diabetes: A Double-Blind Randomized Crossover Trial. Diabetes Care. 2020;43(1):29–36. https://doi.org/10.2337/dc19-0895 (Epub 2019 Oct 1 PMID: 31575640).
Dassau E, Renard E, Place J, Farret A, Pelletier MJ, Lee J, Huyett LM, Chakrabarty A, Doyle FJ 3rd, Zisser HC. Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab. 2017 Dec;19(12):1698–1705. https://doi.org/10.1111/dom.12999. Epub 2017 Jul 6. PMID: 28474383; PMCID: PMC5742859.
Haidar A, Tsoukas MA, Bernier-Twardy S, Yale JF, Rutkowski J, Bossy A, Pytka E, El Fathi A, Strauss N, Legault L. A Novel Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care. 2020;43(3):597–606. https://doi.org/10.2337/dc19-1922 (Epub 2020 Jan 23 PMID: 31974099).
Tagougui S, Taleb N, Molvau J, Nguyen É, Raffray M, Rabasa-Lhoret R. Artificial Pancreas Systems and Physical Activity in Patients with Type 1 Diabetes: Challenges, Adopted Approaches, and Future Perspectives. J Diabetes Sci Technol. 2019 Nov;13(6):1077–1090. https://doi.org/10.1177/1932296819869310. Epub 2019 Aug 13. PMID: 31409125; PMCID: PMC6835182.
Breton MD, Brown SA, Karvetski CH, Kollar L, Topchyan KA, Anderson SM, Kovatchev BP. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014 Aug;16(8):506–11. https://doi.org/10.1089/dia.2013.0333. Epub 2014 Apr 4. PMID: 24702135; PMCID: PMC4116126.
Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, Balliro C, Hillard MA, Nathan DM, Damiano ER. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014 Jul 24;371(4):313–325. https://doi.org/10.1056/NEJMoa1314474. Epub 2014 Jun 15. PMID: 24931572; PMCID: PMC4183762.
Russell SJ, Hillard MA, Balliro C, Magyar KL, Selagamsetty R, Sinha M, Grennan K, Mondesir D, Ekhlaspour L, Zheng H, Damiano ER, El-Khatib FH. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016 Mar;4(3):233–243. https://doi.org/10.1016/S2213-8587(15)00489-1. Epub 2016 Feb 3. Erratum in: Lancet Diabetes Endocrinol. 2018 Mar;6(3):e3. PMID: 26850709; PMCID: PMC4799495
El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, Malkani S, Lock JP, Harlan DM, Clinton P, Frank E, Wilson DM, DeSalvo D, Norlander L, Ly T, Buckingham BA, Diner J, Dezube M, Young LA, Goley A, Kirkman MS, Buse JB, Zheng H, Selagamsetty RR, Damiano ER, Russell SJ. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017 Jan 28;389(10067):369–380. https://doi.org/10.1016/S0140-6736(16)32567-3. Epub 2016 Dec 20. Erratum in: Lancet. 2017 Jan 28;389(10067):368. Erratum in: Lancet. 2017 Feb 4;389(10068):e2. PMID: 28007348; PMCID: PMC5358809.
Haidar A, Rabasa-Lhoret R, Legault L, Lovblom LE, Rakheja R, Messier V, D’Aoust É, Falappa CM, Justice T, Orszag A, Tschirhart H, Dallaire M, Ladouceur M, Perkins BA. Single- and Dual-Hormone Artificial Pancreas for Overnight Glucose Control in Type 1 Diabetes. J Clin Endocrinol Metab. 2016;101(1):214–23. https://doi.org/10.1210/jc.2015-3003 (Epub 2015 Nov 2 PMID: 26523526).
Blauw H, van Bon AC, Koops R, DeVries JH; , on behalf of the PCDIAB consortium. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016 Jul;18(7):671–7. https://doi.org/10.1111/dom.12663. Epub 2016 Apr 25. PMID: 26996542; PMCID: PMC5111773.
Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9(9):555–62. https://doi.org/10.1038/nrendo.2013.138 (Epub 2013 Jul 30 PMID: 23897173).
Bellin MD, Dunn TB. Transplant strategies for type 1 diabetes: whole pancreas, islet and porcine beta cell therapies. Diabetologia. 2020;63(10):2049–56. https://doi.org/10.1007/s00125-020-05184-7 (Epub 2020 Sep 7 PMID: 32894315).
Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350(7):694–705. https://doi.org/10.1056/NEJMra032425 (PMID: 14960745).
Lablanche S, Vantyghem MC, Kessler L, Wojtusciszyn A, Borot S, Thivolet C, Girerd S, Bosco D, Bosson JL, Colin C, Tetaz R, Logerot S, Kerr-Conte J, Renard E, Penfornis A, Morelon E, Buron F, Skaare K, Grguric G, Camillo-Brault C, Egelhofer H, Benomar K, Badet L, Berney T, Pattou F, Benhamou PY; TRIMECO trial investigators. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018 Jul;6(7):527–537. https://doi.org/10.1016/S2213-8587(18)30078-0. Epub 2018 May 15. PMID: 29776895.
Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, Ho S, Worsley D, Fung M, Meneilly G, Begg I, Al Mehthel M, Kondi J, Harris C, Fensom B, Kozak SE, Tong SO, Trinh M, Warnock GL. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–8. https://doi.org/10.1097/TP.0b013e31820437f3 (PMID: 21258272).
Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012 Jun;12(6):1576–83. https://doi.org/10.1111/j.1600-6143.2011.03977.x. Epub 2012 Apr 11. PMID: 22494609; PMCID: PMC3390261
Carlsson PO, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, Goldman T, Barkai U, Avni Y, Westermark GT, Carlbom L, Ahlström H, Eriksson O, Olerud J, Korsgren O. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018 Jul;18(7):1735–1744. https://doi.org/10.1111/ajt.14642. Epub 2018 Feb 2. PMID: 29288549; PMCID: PMC6055594.
Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transplant. 2020;25(5):449–56. https://doi.org/10.1097/MOT.0000000000000794 (PMID: 32773503).
Pathak V, Pathak NM, O’Neill CL, Guduric-Fuchs J, Medina RJ. Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives. Clin Med Insights Endocrinol Diabetes. 2019;3(12):1179551419844521. https://doi.org/10.1177/1179551419844521.PMID:31105434;PMCID:PMC6501476.
Chen S, Du K, Zou C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther. 2020;11(1):275. https://doi.org/10.1186/s13287-020-01793-6.PMID:32641151;PMCID:PMC7346484.
Hwang G, Jeong H, Yang HK, Kim HS, Hong H, Kim NJ, Oh IH, Yim HW. Efficacies of Stem Cell Therapies for Functional Improvement of the β Cell in Patients with Diabetes: A Systematic Review of Controlled Clinical Trials. Int J Stem Cells. 2019;12(2):195–205. https://doi.org/10.15283/ijsc18076.PMID:31022997;PMCID:PMC6657948.
Senior PA, Pettus JH. Stem cell therapies for Type 1 diabetes: current status and proposed road map to guide successful clinical trials. Diabet Med. 2019;36(3):297–307. https://doi.org/10.1111/dme.13846 (Epub 2018 Nov 27 PMID: 30362170).
Memon B, Abdelalim EM. Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors. Cells. 2020;9(2):283. https://doi.org/10.3390/cells9020283.PMID:31979403;PMCID:PMC7072676.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. https://doi.org/10.1126/science.282.5391.1145.Erratum.In:Science1998Dec4;282(5395):1827 (PMID: 9804556).
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019 (PMID: 18035408).
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33. https://doi.org/10.1038/nbt.3033 (Epub 2014 Sep 11 PMID: 25211370).
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–401. https://doi.org/10.1038/nbt1259 (Epub 2006 Oct 19 PMID: 17053790).
Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19(4):429–38. https://doi.org/10.1038/cr.2009.28 (PMID: 19255591).
Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283(46):31601–7. https://doi.org/10.1074/jbc.M806597200 (Epub 2008 Sep 9 PMID: 18782754).
Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, Poh YC, Sintov E, Gürtler M, Pagliuca FW, Peterson QP, Melton DA. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019 May;569(7756):368–373. https://doi.org/10.1038/s41586-019-1168-5. Epub 2019 May 8. PMID: 31068696; PMCID: PMC6903417
Legøy TA, Mathisen AF, Salim Z, Vethe H, Bjørlykke Y, Abadpour S, Paulo JA, Scholz H, Ræder H, Ghila L, Chera S. In vivo Environment Swiftly Restricts Human Pancreatic Progenitors Toward Mono-Hormonal Identity via a HNF1A/HNF4A Mechanism. Front Cell Dev Biol. 2020;25(8):109. https://doi.org/10.3389/fcell.2020.00109.PMID:32161757;PMCID:PMC7052484.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, Ao Z, Warnock GL, Kieffer TJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012 Aug;61(8):2016–29. https://doi.org/10.2337/db11-1711. Epub 2012 Jun 27. PMID: 22740171; PMCID: PMC3402300.
Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Orive G, Hernández RMM, Saenz Del Burgo L, Pedraz JL. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics. 2019;11(11):597. https://doi.org/10.3390/pharmaceutics11110597.PMID:31726670;PMCID:PMC6920807.
Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15768–73. https://doi.org/10.1073/pnas.0906894106. Epub 2009 Aug 31. PMID: 19720998; PMCID: PMC2735559.
Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun. 2016;10(7):11463. https://doi.org/10.1038/ncomms11463.Erratum.In:NatCommun.2016Aug04;7:12379.PMID:27163171;PMCID:PMC4866045.
Chellappan DK, Sivam NS, Teoh KX, Leong WP, Fui TZ, Chooi K, Khoo N, Yi FJ, Chellian J, Cheng LL, Dahiya R, Gupta G, Singhvi G, Nammi S, Hansbro PM, Dua K. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188–200. https://doi.org/10.1016/j.biopha.2018.09.138 (Epub 2018 Oct 2 PMID: 30372820).
Lei P, Ogunade A, Kirkwood KL, Laychock SG, Andreadis ST. Efficient production of bioactive insulin from human epidermal keratinocytes and tissue-engineered skin substitutes: implications for treatment of diabetes. Tissue Eng. 2007;13(8):2119–31. https://doi.org/10.1089/ten.2006.0210 (PMID: 17518716).
Falqui L, Martinenghi S, Severini GM, Corbella P, Taglietti MV, Arcelloni C, Sarugeri E, Monti LD, Paroni R, Dozio N, Pozza G, Bordignon C. Reversal of diabetes in mice by implantation of human fibroblasts genetically engineered to release mature human insulin. Hum Gene Ther. 1999;10(11):1753–62. https://doi.org/10.1089/10430349950017437 (PMID: 10446915).
Alam T, Wai P, Held D, Vakili ST, Forsberg E, Sollinger H. Correction of Diabetic Hyperglycemia and Amelioration of Metabolic Anomalies by Minicircle DNA Mediated Glucose-Dependent Hepatic Insulin Production. PLoS ONE. 2013;8(6):e67515. https://doi.org/10.1371/journal.pone.0067515.PMID:23826312;PMCID:PMC3694888.
Jacovetti C, Jimenez V, Ayuso E, Laybutt R, Peyot ML, Prentki M, Bosch F, Regazzi R. Contribution of Intronic miR-338–3p and Its Hosting Gene AATK to Compensatory β-Cell Mass Expansion. Mol Endocrinol. 2015 May;29(5):693–702. https://doi.org/10.1210/me.2014-1299. Epub 2015 Mar 9. PMID: 25751313; PMCID: PMC5414744.
Insel R, Dunne JL. JDRF’s vision and strategy for prevention of type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl 22):87–92. https://doi.org/10.1111/pedi.12326 (PMID: 27411442).
Clemente-Casares X, Tsai S, Huang C, Santamaria P. Antigen-specific therapeutic approaches in Type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(2):a007773. https://doi.org/10.1101/cshperspect.a007773.PMID:22355799;PMCID:PMC3281592.
Ziegler AG, Achenbach P, Berner R, Casteels K, Danne T, Gündert M, Hasford J, Hoffmann VS, Kordonouri O, Lange K, Elding Larsson H, Lundgren M, Snape MD, Szypowska A, Todd JA, Bonifacio E; GPPAD Study group. Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol. BMJ Open. 2019 Jun 28;9(6):e028578. https://doi.org/10.1136/bmjopen-2018-028578. PMID: 31256036; PMCID: PMC6609035.
Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, Colman PG, Harrison LC. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011 Apr;60(4):1237–45. https://doi.org/10.2337/db10-1360. Epub 2011 Feb 9. PMID: 21307076; PMCID: PMC3064097.
Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002 May 30;346(22):1685–91. https://doi.org/10.1056/NEJMoa012350. PMID: 12037147.
Dunne JL, Triplett EW, Gevers D, Xavier R, Insel R, Danska J, Atkinson MA. The intestinal microbiome in type 1 diabetes. Clin Exp Immunol. 2014;177(1):30–7. https://doi.org/10.1111/cei.12321.PMID:24628412;PMCID:PMC4089152.
Atkinson MA, Chervonsky A. Does the gut microbiota have a role in type 1 diabetes? Early evidence from humans and animal models of the disease. Diabetologia. 2012 Nov;55(11):2868–77. https://doi.org/10.1007/s00125-012-2672-4. Epub 2012 Aug 9. PMID: 22875196; PMCID: PMC3496388.
Assan R, Feutren G, Sirmai J, Laborie C, Boitard C, Vexiau P, Du Rostu H, Rodier M, Figoni M, Vague P, et al. Plasma C-peptide levels and clinical remissions in recent-onset type I diabetic patients treated with cyclosporin A and insulin. Diabetes. 1990;39(7):768–74. https://doi.org/10.2337/diab.39.7.768 (PMID: 2191883).
Haller MJ, Long SA, Blanchfield JL, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Geyer SM, Warnock MV, Miller JL, Atkinson MA, Becker DJ, Baidal DA, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell WE, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes. 2019 Jun;68(6):1267–1276. https://doi.org/10.2337/db19-0057. Epub 2019 Apr 9. PMID: 30967424; PMCID: PMC6610026.
Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, Moran A, Raskin P, Rodriguez H, Schatz DA, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014 Feb;37(2):453–9. https://doi.org/10.2337/dc13-0626. Epub 2013 Sep 11. PMID: 24026563; PMCID: PMC3898764.
Jacobsen LM, Newby BN, Perry DJ, Posgai AL, Haller MJ, Brusko TM. Immune Mechanisms and Pathways Targeted in Type 1 Diabetes. Curr Diab Rep. 2018;18(10):90. https://doi.org/10.1007/s11892-018-1066-5 (PMID: 30168021).
Kanta A, Lyka E, Koufakis T, Zebekakis P, Kotsa K. Prevention strategies for type 1 diabetes: a story of promising efforts and unmet expectations. Hormones (Athens). 2020 May 16. https://doi.org/10.1007/s42000-020-00207-9. Epub ahead of print. PMID: 32415650.
Ricordi C, Clare-Salzler M, Infante M, Baggerly C, Aliano J, McDonnell S, Chritton S. Vitamin D and Omega 3 Field Study on Progression of Type 1 Diabetes. CellR4 Repair Replace Regen Reprogram. 2019;7:e2737. https://doi.org/10.32113/cellr4_20198_2737. Epub 2019 Aug 28. PMID: 31572748; PMCID: PMC6768421.
Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37(6):552–8. https://doi.org/10.1007/BF00403372 (PMID: 7926338).
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000 Oct;23(10):1516–26. https://doi.org/10.2337/diacare.23.10.1516. PMID: 11023146.
Pozzilli P, Manfrini S, Crinò A, Picardi A, Leomanni C, Cherubini V, Valente L, Khazrai M, Visalli N; IMDIAB group. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005 Nov;37(11):680–3. https://doi.org/10.1055/s-2005-870578. PMID: 16308836.
Bi X, Li F, Liu S, Jin Y, Zhang X, Yang T, Dai Y, Li X, Zhao AZ. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Invest. 2017 May 1;127(5):1757–1771. https://doi.org/10.1172/JCI87388. Epub 2017 Apr 4. PMID: 28375156; PMCID: PMC5409789.
Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420–8. https://doi.org/10.1001/jama.298.12.1420.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This review was in accordance with the principles of the Declaration of Helsinki.
Human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boscari, F., Avogaro, A. Current treatment options and challenges in patients with Type 1 diabetes: Pharmacological, technical advances and future perspectives. Rev Endocr Metab Disord 22, 217–240 (2021). https://doi.org/10.1007/s11154-021-09635-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-021-09635-3