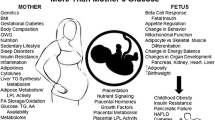
Abstract
Fetal growth is a complex process which depends both on the genetic makeup and intrauterine environment. Maternal nutrition during pregnancy is an important determinant of fetal growth. Adequate nutrient supply is required during pregnancy and lactation for the support of fetal/infant growth and development. Macro- and micronutrients are both important to sustain pregnancy and for appropriate growth of the fetus. While macronutrients provide energy and proteins for fetal growth, micronutrients play a major role in the metabolism of macronutrients, structural and cellular metabolism of the fetus. Discrepancies in maternal diet at different stages of foetal growth / offspring development can have pronounced influences on the health and well-being of the offspring. Indeed intrauterine growth restriction induced by nutrient insult can irreversibly modulate the endocrine/metabolic status of the fetus that leads to the development of adiposity and insulin resistance in its later life. Understanding the role of micronutrients during the development of fetus will provide insights into the probable underlying / associated mechanisms in the metabolic pathways of endocrine related complications. Keeping in view the modernized lifestyle and food habits that lead to the development of adiposity and world burden of obesity, this review focuses mainly on the role of maternal micronutrients in the foetal origins of adiposity.
Similar content being viewed by others
References
Bernstein I, Gabbe SG. Intrauterine growth restriction. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: normal & problem pregnancies. 3rd ed. New York: Churchill Livingstone; 1996. p. 863–886.
Eleftheriades M, Creatsas G, Nicolaides K. Fetal growth restriction and postnatal development. Ann N Y Acad Sci. 2006;1092:319–30.
Neerhof MG. Causes of intrauterine growth restriction. Clin Perinatol. 1995;22:375–85.
Bryan SM, Hindmarsh PC. Normal and abnormal fetal growth. Horm Res. 2006;65 Suppl 3:19–27.
Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–7.
Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–3.
Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–30.
Barker DJ. In utero programming of chronic disease. Clin Sci (Lond). 1998;95:115–28.
Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22.
Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85:1401–6.
de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52 Suppl 1:S5–S15.
Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107.
McAnarney ER. Young maternal age and adverse neonatal outcome. Am J Dis Child. 1987;141:1053–9.
Adelson PL, Frommer MS, Pym MA, Rubin GL. Teenage pregnancy and fertility in New South Wales: an examination of fertility trends, abortion and birth outcomes. Aust J Public Health. 1992;16:238–44.
Cooper LG, Leland NL, Alexander G. Effect of maternal age on birth outcomes among young adolescents. Soc Biol. 1995;42:22–35.
WHO/UNICEF. Low birthweight: country, regional and global estimates. New York, United Nations Children’s Fund and World Health Organization, 2004
Abrams SA. In utero physiology: role in nutrient delivery and fetal development for calcium, phosphorus, and vitamin D. Am J Clin Nutr. 2007;85:604S–7S.
UNICEF: Vitamin and mineral deficiencies: a global progress report. The Micronutrient Initiative and UNICEF, 2004 by Peter Adamson, P&LA, Oxfordshire, UK.
Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71:1295S–303S.
Coursin DB. Vitamin B6 metabolism in infants and children. Vitam Horm. 1964;22:755–86.
Cederberg J, Siman CM, Eriksson UJ. Combined treatment with vitamin E and vitamin C decreases oxidative stress and improves fetal outcome in experimental diabetic pregnancy. Pediatr Res. 2001;49:755–62.
Siman CM, Eriksson UJ. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia. 1997;40:1416–24.
Raman L, Rajalakshmi K, Krishnamachari KA, Sastry JG. Effect of calcium supplementation to undernourished mothers during pregnancy on the bone density of the bone density of the neonates. Am J Clin Nutr. 1978;31:466–9.
Koo WW, Walters JC, Esterlitz J, Levine RJ, Bush AJ, Sibai B. Maternal calcium supplementation and fetal bone mineralization. Obstet Gynecol. 1999;94:577–82.
Linder MC. The biochemistry of copper. New York: Plenum Press; 1991.
Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:257S–64S.
Krachler M, Rossipal E, Micetic-Turk D. Trace element transfer from the mother to the newborn–investigations on triplets of colostrum, maternal and umbilical cord sera. Eur J Clin Nutr. 1999;53:486–94.
Hurley LS. Developmental nutrition. Englewood Cliffs: Prentice-Hall; 1980.
Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995;25:457–63.
Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–23.
Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–44.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6.
Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–10.
Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, Willett WC, Villamor E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr. 2010;92:1446–51.
Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, Keeton D, Petty K, Holick MF, Zhu H. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–11.
Krishnaveni GV, Hill JC, Veena SR, Bhat DS, Wills AK, Karat CL, Yajnik CS, Fall CH. Low plasma vitamin B12 in pregnancy is associated with gestational 'diabesity' and later diabetes. Diabetologia. 2009;52:2350–8.
Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond). 2008;32:1098–104.
Venu L, Harishankar N, Krishna TP, Raghunath M. Does maternal dietary mineral restriction per se predispose the offspring to insulin resistance? Eur J Endocrinol. 2004;151:287–94.
Venu L, Harishankar N, Prasanna Krishna T, Raghunath M. Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age. Diabetologia. 2004;47:1493–501.
Venu L, Kishore YD, Raghunath M. Maternal and perinatal magnesium restriction predisposes rat pups to insulin resistance and glucose intolerance. J Nutr. 2005;135:1353–8.
Venu L, Padmavathi IJ, Kishore YD, Bhanu NV, Rao KR, Sainath PB, Ganeshan M, Raghunath M. Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obesity (Silver Spring). 2008;16:1270–6.
Raghunath M, Venu L, Padmavathi I, Kishore YD, Ganeshan M, Anand Kumar K, Sainath PB, Rao KR. Modulation of macronutrient metabolism in the offspring by maternal micronutrient deficiency in experimental animals. Indian J Med Res. 2009;130:655–65.
Padmavathi IJ, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV, Raghunath M. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol. 2009;94:761–9.
Padmavathi IJN, Rao KR, Venu L, Ganeshan M, Kumar KA, Rao Ch N, Harishankar N, Ismail A, Raghunath M: Chronic maternal dietary chromium restriction modulates visceral adiposity: probable underlying mechanisms. Diabetes. 2010;59:98–104.
Manisha Ganeshan SP, Padmavathi IJN, Venu L, Kishore YD, Anand K, Harishanker N, Srinivasa Rao J, Raghunath M (2011) Maternal manganese restriction increases susceptibility to high fat diet induced dyslipidemia and altered adipose function in WNIN male rat offspring. Exp Diabetes Res (In press)
Anand Kumar K, Padmavathi IJN, Lalitha A, Manisha G, Rao KR, Mahesh Kumar J, Chandak GR, Raghunath M: Chronic maternal vitamin B12 restriction induced changes in the wistar rat offspring are partly correctable by rehabilitation (Abstract). CMR e-journal 2010
Ribot J, Felipe F, Bonet ML, Palou A. Changes of adiposity in response to vitamin A status correlate with changes of PPAR gamma 2 expression. Obes Res. 2001;9:500–9.
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7.
Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–9.
Joss-Moore LA, Wang Y, Campbell MS, Moore B, Yu X, Callaway CW, McKnight RA, Desai M, Moyer-Mileur LJ, Lane RH. Uteroplacental insufficiency increases visceral adiposity and visceral adipose PPARgamma2 expression in male rat offspring prior to the onset of obesity. Early Hum Dev. 2010;86:179–85.
Desbriere R, Vuaroqueaux V, Achard V, Boullu-Ciocca S, Labuhn M, Dutour A, Grino M. 11beta-hydroxysteroid dehydrogenase type 1 mRNA is increased in both visceral and subcutaneous adipose tissue of obese patients. Obesity (Silver Spring). 2006;14:794–8.
Boullu-Ciocca S, Achard V, Tassistro V, Dutour A, Grino M. Postnatal programming of glucocorticoid metabolism in rats modulates high-fat diet-induced regulation of visceral adipose tissue glucocorticoid exposure and sensitivity and adiponectin and proinflammatory adipokines gene expression in adulthood. Diabetes. 2008;57:669–77.
Jazet IM, Pijl H, Meinders AE. Adipose tissue as an endocrine organ: impact on insulin resistance. Neth J Med. 2003;61:194–212.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Fontbonne A, Eschwege E, Cambien F, Richard JL, Ducimetiere P, Thibult N, Warnet JM, Claude JR, Rosselin GE. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989;32:300–4.
Smith U. Impaired ('diabetic') insulin signaling and action occur in fat cells long before glucose intolerance–is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord. 2002;26:897–904.
Koo SI, Norvell JE, Algilani K, Chow J. Effect of marginal zinc deficiency on the lymphatic absorption of [14C]cholesterol. J Nutr. 1986;116:2363–71.
Kim ES, Noh SK, Koo SI. Marginal zinc deficiency lowers the lymphatic absorption of alpha-tocopherol in rats. J Nutr. 1998;128:265–70.
LeBlanc CP, Fiset S, Surette ME, Turgeon O'Brien H, Rioux FM. Maternal iron deficiency alters essential fatty acid and eicosanoid metabolism and increases locomotion in adult guinea pig offspring. J Nutr. 2009;139:1653–9.
Crowe C, Dandekar P, Fox M, Dhingra K, Bennet L, Hanson MA. The effects of anaemia on heart, placenta and body weight, and blood pressure in fetal and neonatal rats. J Physiol. 1995;488(Pt 2):515–9.
Lewis RM, Petry CJ, Ozanne SE, Hales CN. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism. 2001;50:562–7.
Singla PN, Tyagi M, Kumar A, Dash D, Shankar R. Fetal growth in maternal anaemia. J Trop Pediatr. 1997;43:89–92.
Zhang J, Lewis RM, Wang C, Hales N, Byrne CD. Maternal dietary iron restriction modulates hepatic lipid metabolism in the fetuses. Am J Physiol Regul Integr Comp Physiol. 2005;288:R104–11.
Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr. 2000;130:2821–30.
Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–202.
Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54:83–90.
Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–8.
Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44:401–6.
Fu Q, McKnight RA, Yu X, Wang L, Callaway CW, Lane RH. Uteroplacental insufficiency induces site-specific changes in histone H3 covalent modifications and affects DNA-histone H3 positioning in day 0 IUGR rat liver. Physiol Genomics. 2004;20:108–16.
Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–8.
Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–6.
Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond). 2002;103:633–9.
Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, Clough GF, Poston L, Hanson MA. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504.
Gallou-Kabani C, Junien C. Nutritional epigenomics of metabolic syndrome: new perspective against the epidemic. Diabetes. 2005;54:1899–906.
Author information
Authors and Affiliations
Corresponding author
Additional information
All the authors contributed equally.
Rights and permissions
About this article
Cite this article
Rao, K.R., Padmavathi, I.J.N. & Raghunath, M. Maternal micronutrient restriction programs the body adiposity, adipocyte function and lipid metabolism in offspring: A review. Rev Endocr Metab Disord 13, 103–108 (2012). https://doi.org/10.1007/s11154-012-9211-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-012-9211-y