Abstract
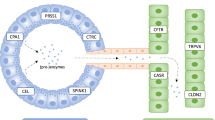
Tropical calcific pancreatitis (TCP) is a subtype of chronic pancreatitis which is unique to tropical regions. Patients present at young age with recurrent abdominal pain, nutritional deficiencies, and insulin-requiring diabetes. For a long time, the aetiology of this disorder was poorly understood. Several environmental factors, such as malnutrition or the consumption of toxic food components such as cyanogenic glycosides, were proposed as pathogenic factors. In the last decade, a major impact on the understanding of the aetiology of TCP has come from genetic studies on hereditary and idiopathic chronic pancreatitis. Genetic alterations in at least five genetic loci are clearly associated with chronic pancreatitis in the Western world. These include alterations in genes coding for trypsinogens, the most abundant digestive enzymes (PRSS1 and PRSS2), the trypsin inhibitor (SPINK1) and the trypsin-degrading enzyme, chymotrypsinogen C (CTRC). In addition, alterations in the cystic fibrosis (CFTR) gene are associated with idiopathic pancreatitis. TCP clinically resembles non-alcoholic chronic pancreatitis of Western countries, suggesting that similar genetic defects might also be of importance in this disease entity. Indeed, alterations in at least two genes, SPINK1 and CTRC, are strongly associated with TCP. The current review focuses on the recent developments in the understanding of the genetic basis of inherited pancreatitis, with special emphasis on TCP.

Similar content being viewed by others
Abbreviations
- FCPD:
-
fibrocalculous pancreatic diabetes
- TCP:
-
tropical calcific pancreatitis
- CP:
-
chronic pancreatitis
- ICP:
-
idiopathic chronic pancreatitis
- CF:
-
cystic fibrosis
- CTRC:
-
chymotrypsinogen C
- PRSS1:
-
cationic trypsinogen
- PRSS2:
-
anionic trypsinogen
- SPINK1:
-
serine protease inhibitor, Kazal type 1
References
Abu-Bakare A, Taylor R, Gill GV, Alberti KGMM. Tropical or malnutrition-related diabetes: a real syndrome? Lancet. 1986;i:1135–8. doi:10.1016/S0140-6736(86)91846-5.
Barman KK, Premlatha G, Mohan V. Tropical chronic pancreatitis. Postgrad Med J. 2003;79:606–15. doi:10.1136/pmj.79.937.606.
Viswanathan M. Pancreatic diabetes in India: an overview. In: Podolsky S, Viswanathan M, editors. Secondary diabetes: the spectrum of diabetic syndromes. New York: Raven; 1980. pp 105–16.
Rao RH. Is tropical diabetes malnutrition related? Diabetes Care. 1993;16:941–5.
Geevarghese PJ, Kumara Pillai V, Joseph MP, Pitchumoni CS. The diagnosis of pancreatogenous diabetes mellitus. J Assoc Phy India. 1962;10:173–80.
Thomas PG, Augustine P, Ramesh H, Rangabashyam N. Observations and surgical management of tropical pancreatitis in Kerala and southern India. World J Surg. 1990;14:32–42. doi:10.1007/BF01670542.
Mittal N, Mehrotra R, Agarwal G, Rajeshwari J, Choudhuri G, Sikora S, et al. The clinical spectrum of fibrocalculous pancreatic diabetes in north India. Natl Med J India. 2002;15:327–31.
Shaper AG. Chronic pancreatic disease and protein malnutrition. Lancet. 1960;i:1223–4. doi:10.1016/S0140-6736(60)91103-X.
McMillan DE, Geevarghese PJ. Dietary cyanide and tropical malnutrition diabetes. Diabetes Care. 1979;2:202–8. doi:10.2337/diacare.2.2.202.
Mohan V, Chari ST, Hitman GA, Suresh S, Madanagopalan N, Ramachandran A, et al. Familial aggregation in tropical fibrocalculous pancreatic diabetes. Pancreas. 1989;4:690–3. doi:10.1097/00006676-198912000-00006.
Yajnik CS, Shelgikar KM. Fibrocalculous pancreatic diabetes in Pune, India. Clinical features and follow-up for 7 years. Diabetes Care. 1993;16:916–21. doi:10.2337/diacare.16.6.916.
Mohan V, Mohan R, Susheela L, Snehalatha C, Bharani G, Mahajan VK, et al. Tropical pancreatic diabetes in south India: heterogeneity in clinical and biochemical profile. Diabetologia. 1985;28:229–32. doi:10.1007/BF00282238.
Mohan V, Premalatha G, Padma A, Chari ST, Pitchumoni CS. Fibrocalculous pancreatic diabetes. Long-term survival analysis. Diabetes Care. 1996;19:1274–8. doi:10.2337/diacare.19.11.1274.
Bhatia E, Baijal SS, Kumar R, Choudhuri G. Exocrine pancreatic and β-cell function in malnutrition related diabetes among North Indians. Diabetes Care. 1995;18:1174–8. doi:10.2337/diacare.18.8.1174.
Yajnik CS, Shelgikar KM, Sahasrabudhe RA, Naik SS, Pai VR, Alberti KGMM, et al. The spectrum of pancreatic exocrine and endocrine (beta cell) function in tropical calcific pancreatitis. Diabetologia. 1995;33:417–21. doi:10.1007/BF00404091.
Mohan V, Barman KK, Rajan VS, Chari ST, Deepa R. Natural history of endocrine failure in tropical chronic pancreatitis: a longitudinal follow-up study. J Gastroenterol Hepatol. 2005;20:1927–34. doi:10.1111/j.1440-1746.2005.04068.x.
Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, Dimagno EP. The different courses of early- and late-onset idiopathic and alcoholic pancreatitis. Gastroenterology. 1994;107:1481–7.
Balaji LN, Tandon BN, Tandon RK, Banks PA. Prevalence and clinical features of chronic pancreatitis in Southern India. Int J Pancreatol. 1994;15:29–34.
Jyotsana VP, Singh SK, Gopal D, Unnikrishnan AG, Agrawal NK, Singh SK, et al. Clinical and biochemical profiles of young diabetics in North-Eastern India. J Assoc Phy India. 2002;50:1130–4.
Bhatia V, Arya V, Dabadghao P, Balasubramanian K, Sharma K, Verghese N, et al. Etiology and outcome of childhood and adolescent diabetes mellitus in North India. J Pediatr Endocrinol Metab. 2004;17:993–9.
Garg PK, Tandon RK. Survey on chronic pancreatitis in the Asia-Pacific region. J Gastroenterol Hepatol. 2004;19:998–1004. doi:10.1111/j.1440-1746.2004.03426.x.
Balakrishnan V, Nair P, Radhakrishnan L, Narayanan VA. Tropical pancreatitis-a distinct entity or merely a type of chronic pancreatitis? Indian J Gastroenterol. 2006;25:74–81.
Cyanogenic glycosides in cassava and bamboo shoots. A human health risk assessment. Technical report series no. 28. Food Standards Australia New Zealand July 2004.
Teuscher T, Baillod P, Rosman JB, Teuscher A. Absence of diabetes in a rural West African population with a high carbohydrate/cassava diet. Lancet. 1987;i:765–8. doi:10.1016/S0140-6736(87)92797-8.
Mathangi DC, Deepa R, Mohan V, Govindarajan M, Namasivayam A. Long-term ingestion of cassava (tapioca) does not produce diabetes or pancreatitis in the rat model. Int J Pancreatol. 2000;27:203–8. doi:10.1385/IJGC:27:3:203.
Sandhyamani S, Vijayakumari A, Balaraman Nair M. Bonnet monkey model for pancreatic changes in induced malnutrition. Pancreas. 1999;18:84–95. doi:10.1097/00006676-199901000-00011.
Chiari H. Über Selbstverdauung des menschlichen Pankreas. Z Heilkunde. 1996;17:69–96.
Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–5. doi:10.1038/ng1096-141.
Sahin-Tóth M, Tóth M. Gain-of-function mutations associated with hereditary pancreatitis enhance autoactivation of human cationic trypsinogen. Biochem Biophys Res Commun. 2000;278:286–9. doi:10.1006/bbrc.2000.3797.
Teich N, Rosendahl J, Tóth M, Mössner J, Sahin-Tóth M. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Hum Mutat. 2006;27:721–30. doi:10.1002/humu.20343.
Teich N, Le Maréchal C, Kukor Z, Caca K, Witzigmann H, Chen JM, et al. Interaction between trypsinogen isoforms in genetically determined pancreatitis: mutation E79K in cationic trypsin (PRSS1) causes increased transactivation of anionic trypsinogen (PRSS2). Hum Mutat. 2004;23:22–31. doi:10.1002/humu.10285.
Comfort MW, Steinberg AG. Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology. 1952;21:54–63.
Witt H, Luck W, Becker M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology. 1999;117:7–10. doi:10.1016/S0016-5085(99)70543-3.
Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem. 2006;281:11879–86. doi:10.1074/jbc.M600124200.
Le Maréchal C, Masson E, Chen JM, Morel F, Ruszniewski P, Levy P, et al. Hereditary pancreatitis caused by triplication of the trypsinogen locus. Nat Genet. 2006;38:1372–4. doi:10.1038/ng1904.
Archer H, Jura N, Keller J, Jacobson M, Bar-Sagi D. A mouse model of hereditary pancreatitis generated by transgenic expression of R122H trypsinogen. Gastroenterology. 2006;131:1844–55. doi:10.1053/j.gastro.2006.09.049.
Bhatia E, Choudhuri G, Sikora SS, Landt O, Kage A, Becker M, et al. Tropical calcific pancreatitis: strong association with SPINK1 trypsin inhibitor mutations. Gastroenterology. 2002;123:1020–5. doi:10.1053/gast.2002.36028.
Chandak GR, Idris MM, Reddy DN, Mani KR, Bhaskar S, Rao GV, et al. Absence of PRSS1 mutations and association of SPINK1 trypsin inhibitor mutations in hereditary and non-hereditary chronic pancreatitis. Gut. 2004;53:723–8. doi:10.1136/gut.2003.026526.
Masson E, Le Maréchal C, Chandak GR, Lamoril J, Bezieau S, Mahurkar S, et al. Trypsinogen copy number mutations in patients with idiopathic chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6:82–8. doi:10.1016/j.cgh.2007.10.004.
Witt H, Sahin-Tóth M, Landt O, Chen JM, Kähne T, Drenth JP, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668–73. doi:10.1038/ng1797.
Santhosh S, Witt H, te Morsche RH, Nemoda Z, Molnár T, Pap A, et al. A loss of function polymorphism (G191R) of anionic trypsinogen (PRSS2) confers protection against chronic pancreatitis. Pancreas. 2008;36:317–20.
Santhosh S, Chacko A, Dutta AK, Bhatia E, Witt H, te Morsche RHM, et al. Divergent roles of SPINK1 and PRSS2 variants in tropical calcific pancreatitis. Pancreatology. 2008 (in press).
Idris MM, Bhaskar S, Reddy DN, Mani KR, Rao GV, Singh L, et al. Mutations in anionic trypsinogen gene are not associated with tropical calcific pancreatitis. Gut. 2005;54:728–9. doi:10.1136/gut.2004.055335.
Laskowski M, Wu FC. Temporary inhibition of trypsin. J Biol Chem. 1953;204:797–805.
Witt H, Luck W, Hennies HC, Claßen M, Kage A, Laß U, et al. Mutations in the gene encoding mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–6. doi:10.1038/76088.
Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi:10.1053/j.gastro.2007.03.001.
Kuwata K, Hirota M, Shimizu H, Nakae M, Nishihara S, Takimoto A, et al. Functional analysis of recombinant pancreatic secretory trypsin inhibitor protein with amino-acid substitution. J Gastroenterol. 2002;37:928–34. doi:10.1007/s005350200156.
Király O, Wartmann T, Sahin-Tóth M. Missense mutations in pancreatic secretory trypsin inhibitor (SPINK1) cause intracellular retention and degradation. Gut. 2007;56:1433–8. doi:10.1136/gut.2006.115725.
Boulling A, Le Maréchal C, Trouvé P, Raguénès O, Chen JM, Férec C. Functional analysis of pancreatitis-associated missense mutations in the pancreatic secretory trypsin inhibitor (SPINK1) gene. Eur J Hum Genet. 2007;15:936–42. doi:10.1038/sj.ejhg.5201873.
Király O, Boulling A, Witt H, Le Maréchal C, Chen JM, Rosendahl J, et al. Signal peptide variants that impair secretion of pancreatic secretory trypsin inhibitor (SPINK1) cause autosomal dominant hereditary pancreatitis. Hum Mutat. 2007;28:469–76. doi:10.1002/humu.20471.
Nathan JD, Romac J, Peng RY, Peyton M, Macdonald RJ, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–27. doi:10.1053/j.gastro.2004.11.052.
Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129:696–705.
Ohmuraya M, Hirota M, Araki K, Baba H, Yamamura K. Enhanced trypsin activity in pancreatic acinar cells deficient for serine protease inhibitor kazal type 3. Pancreas. 2006;33:104–6. doi:10.1097/01.mpa.0000226889.86322.9b.
Chandak GR, Idris MM, Reddy DN, Bhaskar S, Sriram PV, Singh L. Mutations in the pancreatic secretory trypsin inhibitor gene (PSTI/SPINK1) rather than the cationic trypsinogen gene (PRSS1) are significantly associated with tropical calcific pancreatitis. J Med Genet. 2002;39:347–51. doi:10.1136/jmg.39.5.347.
Hassan Z, Mohan V, Ali L, Allotey R, Barakat K, Faruque MO, et al. SPINK1 is a susceptibility gene for fibrocalculous pancreatic diabetes in subjects from the Indian subcontinent. Am J Hum Genet. 2002;71:964–8. doi:10.1086/342731.
Schneider A, Suman A, Rossi L, Barmada MM, Beglinger C, Parvin S, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology. 2002;123:1026–30. doi:10.1053/gast.2002.36059.
Witt H, Luck W, Becker M, Böhmig M, Kage A, Truninger K, et al. Mutation in the SPINK1 trypsin inhibitor gene, alcohol use, and chronic pancreatitis. JAMA. 2001;285:2716–7. doi:10.1001/jama.285.21.2716-a.
Witt H. Chronic pancreatitis and cystic fibrosis. Gut. 2003;52(Suppl 2):ii31–41. doi:10.1136/gut.52.suppl_2.ii31.
Kaneko K, Nagasaki Y, Furukawa T, Mizutamari H, Sato A, Masamune A, et al. Analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene mutations in Japanese patients with chronic pancreatitis. J Hum Genet. 2001;46:293–7. doi:10.1007/s100380170082.
Kume K, Masamune A, Mizutamari H, Kaneko K, Kikuta K, Satoh M, et al. Mutations in the serine protease inhibitor Kazal Type 1 (SPINK1) gene in Japanese patients with pancreatitis. Pancreatology. 2005;5:354–60. doi:10.1159/000086535.
Kume K, Masamune A, Kikuta K, Shimosegawa T. [-215G>A; IVS3+2T>C] mutation in the SPINK1 gene causes exon 3 skipping and loss of the trypsin binding site. Gut. 2006;55:1214. doi:10.1136/gut.2006.095752.
Snabboon T, Plengpanich W, Sridama V, Sunthornyothin S, Suwanwalaikorn S, Khovidhunkit W. A SPINK1 gene mutation in a Thai patient with fibrocalculous pancreatic diabetes. Southeast Asian J Trop Med Public Health. 2006;37:559–62.
Tomomura A, Fukushige T, Noda T, Noikura T, Saheki T. Serum calcium-decreasing factor (caldecrin) from porcine pancreas has proteolytic activity which has no clear connection with the calcium decrease. FEBS Lett. 1992;301:277–81. doi:10.1016/0014-5793(92)80256-G.
Szmola R, Sahin-Tóth M. Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht's enzyme Y. Proc Natl Acad Sci U S A. 2007;104:11227–32. doi:10.1073/pnas.0703714104.
Rinderknecht H, Adham NF, Renner IG, Carmack C. A possible zymogen self-destruct mechanism preventing pancreatic autodigestion. Int J Pancreatol. 1988;3:33–44.
Rosendahl J, Witt H, Szmola R, Bhatia E, Ózsvári B, Landt O, et al. CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet. 2008;40:78–82. doi:10.1038/ng.2007.44.
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi:10.1126/science.2475911.
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi:10.1126/science.2570460.
Riordan JR. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–30. doi:10.1146/annurev.ph.55.030193.003141.
Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645–52. doi:10.1056/NEJM199809033391001.
Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–65. doi:10.1056/NEJM199809033391002.
Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121:1310–9. doi:10.1053/gast.2001.29673.
Audrézet MP, Chen JM, Le Maréchal C, Ruszniewski P, Robaszkiewicz M, Raguenes O, et al. Determination of the relative contribution of three genes-the cystic fibrosis transmembrane conductance regulator gene, the cationic trypsinogen gene, and the pancreatic secretory trypsin inhibitor gene-to the etiology of idiopathic chronic pancreatitis. Eur J Hum Genet. 2002;10:100–6. doi:10.1038/sj.ejhg.5200786.
Bhatia E, Durie P, Zielenski J, Lam D, Sikora SS, Choudhuri G, et al. Mutations in the cystic fibrosis transmembrane regulator gene in patients with tropical calcific pancreatitis. Am J Gastroenterol. 2000;95:3658–9. doi:10.1111/j.1572-0241.2000.03400.x.
Figarella C, Miszczuk-Jamska B, Barrett A. Possible lysosomal activation of pancreatic zymogens: activation of both human trypsinogens by cathepsin B and spontaneous acid activiation of human trypsinogen-1. Biol Chem Hoppe Seyler. 1988;369:293–8.
Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457–67.
Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–81. doi:10.1172/JCI9411.
Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991;87:362–6. doi:10.1172/JCI114995.
Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304–10. doi:10.1016/S0016-5085(97)70108-2.
Mahurkar S, Idris MM, Reddy DN, Bhaskar S, Rao GV, Thomas V, et al. Association of cathepsin B gene polymorphisms with tropical calcific pancreatitis. Gut. 2006;55:1270–5. doi:10.1136/gut.2005.087403.
Montalto G, Multigner L, Sarles H, De Caro A. Organic matrix of pancreatic stones associated with nutritional pancreatitis. Pancreas. 1988;3:263–8. doi:10.1097/00006676-198805000-00004.
Multigner L, De Caro A, Lombardo D, Campese D, Sarles H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochem Biophys Res Commun. 1983;110:69–74. doi:10.1016/0006-291X(83)91261-5.
Bernard JP, Adrich Z, Montalto G, De Caro A, De Reggi M, Sarles H, et al. Inhibition of nucleation and crystal growth of calcium carbonate by human lithostathine. Gastroenterology. 1992;103:1277–84.
Giorgi D, Bernard JP, Rouquier S, Iovanna J, Sarles H, Dagorn JC. Secretory pancreatic stone protein messenger RNA. Nucleotide sequence and expression in chronic calcifying pancreatitis. J Clin Invest. 1989;84:100–6. doi:10.1172/JCI114128.
Bimmler D, Graf R, Scheele GA, Frick TW. Pancreatic stone protein (lithostathine), a physiologically relevant pancreatic calcium carbonate crystal inhibitor? J Biol Chem. 1997;272:3073–82. doi:10.1074/jbc.272.5.3073.
Hawrami K, Mohan V, Bone A, Hitman GA. Analysis of islet regenerating (reg) gene polymorphisms in fibrocalculous pancreatic diabetes. Pancreas. 1997;14:122–5. doi:10.1097/00006676-199703000-00003.
Banchuin N, Boonyasrisawat W, Pulsawat P, Vannasaeng S, Deerochanawong C, Sriussadaporn S, et al. No abnormalities of reg1 alpha and reg1 beta gene associated with diabetes mellitus. Diabetes Res Clin Pract. 2002;55:105–11. doi:10.1016/S0168-8227(01)00321-7.
Boonyasrisawat W, Pulsawat P, Yenchitsomanus PT, Vannasaeng S, Pramukkul P, Deerochanawong C, et al. Analysis of the reg1alpha and reg1beta gene transcripts in patients with fibrocalculous pancreatopathy. Southeast Asian J Trop Med Public Health. 2002;33:365–72.
Mahurkar S, Bhaskar S, Reddy DN, Rao GV, Chandak GR. Comprehensive screening for reg1alpha gene rules out association with tropical calcific pancreatitis. World J Gastroenterol. 2007;13:5938–43.
Leung PS, Chappell MC. A local pancreatic renin-angiotensin system: endocrine and exocrine roles. Int J Biochem Cell Biol. 2003;35:834–46.
Ip SP, Kwan PC, Williams CH, Pang S, Hooper NM, Leung PS. Changes of angiotensin-converting enzyme activity in the pancreas of chronic hypoxia and acute pancreatitis. Int J Biochem Cell Biol. 2003;35:944–54. doi:10.1016/S1357-2725(02)00181-4.
Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–9. doi:10.1053/gast.2003.50147.
Yamada T, Kuno A, Masuda K, Ogawa K, Sogawa M, Nakamura S, et al. Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther. 2003;307:17–23. doi:10.1124/jpet.103.053322.
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half of variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi:10.1172/JCI114844.
Bhaskar S, Reddy DN, Mahurkar S, Rao GV, Singh L, Chandak GR. Lack of significant association of an insertion/deletion polymorphism in the angiotensin converting enzyme (ACE) gene with tropical calcific pancreatitis. BMC Gastroenterol. 2006;6:42. doi:10.1186/1471-230X-6-42.
Hendy GN, D'Souza-Li L, Yang B, Canaff L, Cole DE. Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2000;16:281–96. doi:10.1002/1098-1004(200010)16:4<281::AID-HUMU1>3.0.CO;2-A.
Murugaian EE, Premkumar RM, Radhakrishnan L, Vallath B. Novel mutations in the calcium sensing receptor gene in tropical chronic pancreatitis in India. Scand J Gastroenterol. 2008;43(1):117–21.
Felderbauer P, Hoffmann P, Einwächter H, Bulut K, Ansorge N, Schmitz F, et al. A novel mutation of the calcium sensing receptor gene is associated with chronic pancreatitis in a family with heterozygous SPINK1 mutations. BMC Gastroenterol. 2003;3:34. doi:10.1186/1471-230X-3-34.
Felderbauer P, Klein W, Bulut K, Ansorge N, Dekomien G, Werner I, et al. Mutations in the calcium-sensing receptor: a new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol. 2006;41:343–8. doi:10.1080/00365520510024214.
Kambo PK, Hitman GA, Mohan V, Ramachandran A, Snehalatha C, Suresh S, et al. The genetic predisposition to fibrocalculous pancreatic diabetes. Diabetologia. 1989;32:45–51. doi:10.1007/BF00265403.
Sanjeevi CB, Kanungo A, Shtauvere A, Samal KC, Tripathi BB. Association of HLA class II alleles with different subgroups of diabetes mellitus in Eastern India identify different associations with IDDM and malnutrition-related diabetes. Tissue Antigens. 1999;54:83–7. doi:10.1034/j.1399-0039.1999.540109.x.
Chowdhury ZM, McDermott MF, Davey S, Hassan Z, Sinnott PJ, Hemmatpour SK, et al. Genetic susceptibility to fibrocalculous pancreatic diabetes in Bangladeshi subjects: a family study. Genes Immun. 2002;3:5–8. doi:10.1038/sj.gene.6363814.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial associations or conflicts of interest: None
Rights and permissions
About this article
Cite this article
Witt, H., Bhatia, E. Genetic aspects of tropical calcific pancreatitis. Rev Endocr Metab Disord 9, 213–226 (2008). https://doi.org/10.1007/s11154-008-9088-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-008-9088-y