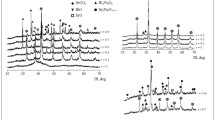
A single-phase material based on the complex oxide Ca2Fe2O5 doped with bismuth was synthesized by glycine-nitrate combustion. Characterization by x-ray diffractometry, helium pycnometry, scanning electron microscope, and elemental analysis showed that the material was isostructural to brownmillerite. The optimum temperature for the synthesis of the material was about 650°C and the material had thermal stability over a wide temperature range according to results of complex thermal analysis together with mass spectrometry. The sintering temperature and thermal expansion coefficient of the material were determined using a dilatometry method.





Similar content being viewed by others
References
A. L. Shaula, Y. V. Pivak, J. C. Waerenborgh, et al., “Ionic conductivity of brownmillerite-type calcium ferrite under oxidizing conditions,” Solid State Ionics, 177, 2923 – 2930 (2006); DOI: https://doi.org/10.1016/j.ssi.2006.08.030.
D. S. Vavilapalli, K. Srikanti, R. Mannam, et al., “Photoactive brownmillerite multiferroic KBiFe2O5 and its potential application in sunlight-driven photocatalysis,” ACS Omega, 3, 16643 – 16650 (2018); DOI: https://doi.org/10.1021/acsomega.8b01744.
B. F. Amorim, M. A. Morales, F. Bohn, et al., “Synthesis of stoichiometric Ca2Fe2O5 nanoparticles by high-energy ball milling and thermal annealing,” Phys. B, 488, 43 – 48 (2016); DOI: https://doi.org/10.1016/j.physb.2016.01.029.
B. V. Beznosikov and K. S. Aleksandrov, Brownmillerite-type Crystals. Crystal Chemistry, Prediction of New Compounds, Preprint No. 840F, Ross. Akad. Nauk, Sib. Otd., Inst. Fiz. im. L. V. Kirenskogo, IF SO RAN, Krasnoyarsk, 2007, 27 pp.
H. Kruger, V. Kahlenberg, V. Petricek, et al., “High-temperature structural phase transition in Ca2Fe2O5 studied by in-situ x-ray diffraction and transmission electron microscopy,” J. Solid State Chem., 182, 1515 – 1523 (2009); DOI:https://doi.org/10.1016/j.jssc.2009.03.027.
C. B. Azzoni, M. C. Mozzati, V. Massarotti, et al., “New insights into the magnetic properties of Ca2Fe2O5 ferrite,” Solid State Sci., 9, 515 – 520 (2007); DOI:https://doi.org/10.1016/j.solidstatesciences.2007.04.013.
S. Dhankhar, G. Bhalerao, K. Baskar, and Sh. Singh, “Synthesis and characterization of polycrystalline brownmillerite cobalt doped Ca2Fe2O5,” AIP Conf. Proc., 1731, 140032 (2016); DOI: https://doi.org/10.1063/1.4948198.
N. A. Lomanova, M. V. Tomkovich, A. V. Osipov, et al., “Formation of Bi1–xCaxFeO3–δ nanocrystals under the glycine-nitrate combustion conditions,” Zh. Obshch. Khim., 89(9), 1448 – 1456 (2019); DOI: https://doi.org/10.1134/S0044460X19090191.
V. V. Gusarov, “The thermal effect of melting in polycrystalline systems” Thermochim. Acta, 256(2), 467 – 472 (1995); DOI: https://doi.org/10.1016/0040-6031(94)01993-Q.
N. A. Lomanova and V. V. Gusarov, “Influence of synthesis temperature on BiFeO3 nanoparticles formation,” Nanosyst.: Phys., Chem., Math., 4(5), 696 – 705 (2013).
A. N. Kovalenko and E. A. Tugova, “Thermodynamics and kinetics of non-autonomous phase formation in nanostructured materials with variable functional properties,” Nanosyst.: Phys., Chem., Math., 9(5), 641 – 662 (2018); DOI: https://doi.org/10.17586/2220-8054-2018-9-5-641-662.
O. N. Karpov, M. V. Tomkovich, and E. A. Tugova, “Formation of Nd1–xBixFeO3 nanocrystals under conditions of glycine-nitrate synthesis,” Zh. Obshch. Khim., 88(10), 1692 – 1698 (2018); DOI: https://doi.org/10.1134/S0044460X18100177.
R Patel and P. Sawadh, “Tunable multiferroic properties of cerium doped bismuth ferrite,” Nanosyst.: Phys., Chem., Math., 10(3), 255 – 265 (2019); DOI: https://doi.org/10.17586/2220-8054-2019-10-3-255-265.
N. A. Lomanova, A. V. Osipov, and V. L. Ugolkov, “Production of nanocrystalline ceramics based on perovskite-like oxides Bi1–xSrxFeO3–δ,” Nov. Ogneupory, No. 10, 33 – 37 (2019).
O. V. Almjasheva, N. A. Lomanova, V. I. Popkov, et al., “The minimum size of oxide nanocrystals: phenomenological thermodynamic vs crystal-chemical approaches,” Nanosyst.: Phys., Chem., Math., 10(4), 428 – 437 (2019); DOI: https://doi.org/10.17586/2220-8054-2019-10-4-428-437.
R. D. Shannon, “Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides,” Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 32, 751 – 757 (1976).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 4, pp. 36 – 40, April, 2020.
Rights and permissions
About this article
Cite this article
Lomanova, N.A. Synthesis of Oxide-Based Materials with the Brownmillerite Structure of the CaO–Bi2O3–Fe2O3 System. Refract Ind Ceram 61, 207–210 (2020). https://doi.org/10.1007/s11148-020-00457-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-020-00457-5