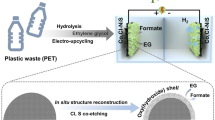
Abstract
Terephthalic acid (TPA) was recovered by alkaline hydrolysis of waste polyethylene terephthalate (PET) bottles. TPA recovered was used to prepare Sn-based catalyst by solvothermal method. For comparison, commercial TPA was also used to prepare Sn-based catalyst. The catalysts were used for the electrochemical reduction of CO2 to formate. Both the catalysts were found to possess nanoflake morphology, and were highly porous in nature with Brunauer–Emmett–Teller (BET) surface area of 211.4 and 239.4 m2/g for commercial and PET derived TPA-based Sn catalysts, respectively. The catalyst made from the waste PET bottles derived TPA was found to be more effective than the one obtained from the commercial TPA. The Faradaic efficiency obtained for the formation of formate was 68.4% at potential − 1.8 V (vs Ag/AgCl) and current density of 13.5 mA/cm2, when catalyst was synthesized using waste PET derived TPA. High activity of the electrocatalysts was attributed to higher capacitance, and lower resistance of the catalysts.
Graphical abstract







Similar content being viewed by others
References
Dechamps P (2023) The IEA world energy outlook 2022—a brief analysis and implications. Eur Energy Clim J 11:100–103
Di Y, Chen Y, Cao Y, Cui X, Liu Y, Zhou C, Di Y (2024) A terbium(III)-organic framework for high catalytic performance on cycloaddition of CO2 with epoxides under mild condition. Catal Lett 154:974–981
Yaashikaa PR, Kumar PS, Varjani SJ, Saravanan A (2019) A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J CO2 Util 33:131–147
Alok A, Shrestha R, Ban S, Devkota S, Uprety B, Joshi R (2022) Technological advances in the transformative utilization of CO2 to value-added products. J Environ Chem Eng 10:106922–106945
Liu S, Su Q, Fu M, Deng L, Wang Y, Dong L, Liu Y, Ma X, Cheng W (2023) Core-shell dispersed polymeric ionic liquids as efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal Lett 153:2429–2442
Liu Q, Ding J, Wang R, Zhong Q (2023) FeZnK/SAPO-34 catalyst for efficient conversion of CO2 to light olefins. Catal Lett 153:54–61
Saeidi S, Amin NAS, Rahimpour MR (2014) Hydrogenation of CO2 to value-added products—a review and potential future developments. J CO2 Util 5:66–81
Kumar P, Srivastava VC, Shukla K, Gläser R, Mishra IM (2016) Dimethyl carbonate synthesis from carbon dioxide using ceria-zirconia catalysts prepared using a templating method: characterization, parametric optimization and chemical equilibrium modeling. RSC Adv 6:110235–110246
Kumawat AS, Sarkar A (2017) Comparative study of carbon supported Pb, Bi and Sn catalysts for electroreduction of carbon dioxide in alkaline medium. J Electrochem Soc 164:H1112–H1120
Quan Y, Zhu J, Zheng G (2021) Electrocatalytic reactions for converting CO2 to value-added products. Small Sci 1:2100043–2100058
Jouny M, Luc W, Jiao F (2018) General techno-economic analysis of CO2 electrolysis systems. Ind Eng Chem Res 57:2165–2177
Agarwal AS, Zhai Y, Hill D, Sridhar N (2011) The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility. ChemSusChem 4:1301–1310
Li H, Oloman C (2005) The electro-reduction of carbon dioxide in a continuous reactor. J Appl Electrochem 35:955–965
Zhang W, Jin Z, Chen Z (2022) Rational-designed principles for electrochemical and photoelectrochemical upgrading of CO2 to value-added chemicals. Adv Sci 9:2105204–2105234
Van Phuc T, Kang SG, Chung JS, Hur SH (2021) Highly CO selective Ca and Zn hybrid metal-organic framework electrocatalyst for the electrochemical reduction of CO2. Curr Appl Phys 27:31–37
Dinh CT, Burdyny T, Kibria MG, Seifitokaldani A, Gabardo CM, García de Arquer FP, Kiani A, Edwards JP, De Luna P, Bushuyev OS, Zou C (2018) CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360:783–787
Back S, Kim H, Jung Y (2015) Selective heterogeneous CO2 electroreduction to methanol. ACS Catal 5:965–971
Peterson AA, Nørskov JK (2012) Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J Phys Chem Lett 3:251–258
Wang H, Hao Y, Sun Y, Pan J, Hu F, Kai D, Peng S (2023) Size control of Zn, N-doped carbon supported copper nanoparticles for effective and selective CO2 electroreduction. Catal Lett 153:2115–2124
Aniruddha R, Sreedhar I, Reddy BM (2020) MOFs in carbon capture-past, present and future. J CO2 Util 42:101297–101324
Chowdhury A, Bhan C, Peela NR, Golder AK (2022) A tunable bioinspired process of SnO2 NPs synthesis for electrochemical CO2-into-formate conversion. J CO2 Util 66:102263–102275
Shen H, Jin H, Li H, Wang H, Duan J, Jiao Y, Qiao SZ (2023) Acidic CO2-to-HCOOH electrolysis with industrial-level current on phase engineered tin sulfide. Nat Commun 14:2843–2853
Zhang W, Xu C, Hu Y, Yang S, Ma L, Wang L, Zhao P, Wang C, Ma J, Jin Z (2020) Electronic and geometric structure engineering of bicontinuous porous Ag-Cu nanoarchitectures for realizing selectivity−tunable electrochemical CO2 reduction. Nano Energy 73:104796–104808
Zhang W, Liu J, Jin Z (2023) Controlled preparation of cross-linked SnO2 hollow nanotube networks for electrocatalytic CO2 reduction. ACS Appl Nano Mater 6:11802–11809
Yang S, Jiang M, Zhang W, Hu Y, Liang J, Wang Y, Tie Z, Jin Z (2023) In situ structure refactoring of bismuth nanoflowers for highly selective electrochemical reduction of CO2 to formate. Adv Funct Mater 33:2301984–2301993
Hu Y, Liang J, Gu Y, Yang S, Zhang W, Tie Z, Ma J, Jin Z (2023) Sandwiched epitaxy growth of 2D single-crystalline hexagonal bismuthene nanoflakes for electrocatalytic CO2 reduction. Nano Lett 23:10512–10521
Nisticò R (2020) Polyethylene terephthalate (PET) in the packaging industry. Polym Test 90:106707–106725
Shi K, Guo L, Zheng R, Wang H, Chen Y (2022) Preparation of diacid comprising ionic liquid catalyst and its application in catalytic degradation of PET. Catal Lett 152:1182–1193
Clausen HF, Poulsen RD, Bond AD, Chevallier MAS, Iversen BB (2005) Solvothermal synthesis of new metal organic framework structures in the zinc-terephthalic acid-dimethyl formamide system. J Solid State Chem 178:3342–3351
Singh S, Sharma S, Umar A, Mehta SK, Bhatti M, Kansal SK (2018) Recycling of waste poly(ethylene terephthalate) bottles by alkaline hydrolysis and recovery of pure nanospindle-shaped terephthalic acid. J Nanosci Nanotechnol 18:5804–5809
Mishra AK, Moorthy JN (2017) Mechanochemical catalytic oxidations in the solid state with in situ-generated modified IBX from 3,5-di-tert-butyl-2-iodobenzoic acid (DTB-IA)/oxone. Org Chem Front 4:343–349
Cosimbescu L, Merkel DR, Darsell J, Petrossian G (2021) Simple but tricky: investigations of terephthalic acid purity obtained from mixed PET waste. Ind Eng Chem Res 60:12792–12797
Elmas Kimyonok AB, Ulutürk M (2016) Determination of the thermal decomposition products of terephthalic acid by using Curie-point pyrolyzer. J Energy Mater 34:113–122
Wang BJ, Ma SY, Pei ST, Xu XL, Cao PF, Zhang JL, Zhang R, Xu XH, Han T (2020) High specific surface area SnO2 prepared by calcining Sn-MOFs and their formaldehyde-sensing characteristics. Sens Actuators B 321:128560–128568
Lu X, Mao Q, Chen Y, Bao L, Tong L, Xiong Q, Qin H, Pan H, Ji Z (2018) A novel oxygen vacancy introduced microstructural reconstruction of SnO2-graphene nanocomposite: demonstration of enhanced electrochemical performance for sodium storage. Electrochim Acta 282:351–361
Wan N, Lu X, Wang Y, Zhang W, Bai Y, Hu YS, Dai S (2016) Improved Li storage performance in SnO2 nanocrystals by a synergetic doping. Sci Rep 6:18978–18989
Deng Z, Zhang Y, Xu D, Zi B, Zeng J, Lu Q, Xiong K, Zhang J, Zhao J, Liu Q (2022) Ultrasensitive formaldehyde sensor based on SnO2 with rich adsorbed oxygen derived from a metal organic framework. ACS Sens 7:2577–2588
Tan D, Lee W, Kim YE, Ko YN, Youn MH, Jeon YE, Hong J, Jeong SK, Park KT (2020) SnO2/ZnO composite hollow nanofiber electrocatalyst for efficient CO2 reduction to formate. ACS Sustain Chem Eng 8:10639–10645
Acknowledgements
We acknowledge SRMIST (Chennai, India), Alagappa University (Karaikudi, India), Annamalai University (Chidambaram, India), and National College (Tiruchirappalli, India) for providing various instrumentation facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shukla, S., Karvembu, R. Synthesis of Sn-based nanocomposites using waste polyethylene terephthalate (PET) for the electrochemical reduction of CO2 to formate. Reac Kinet Mech Cat (2024). https://doi.org/10.1007/s11144-024-02623-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11144-024-02623-z