Abstract
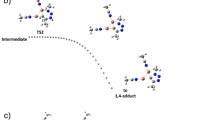
A complementary catalytic system was investigated for the [3 + 2] cycloaddition of azides and halo-alkynes. These are based on three copper complexes with [CuX (PPh3)]; X = I, Br, Cl, which are active at low metal loadings (the PPh3 system). The computational MN12-L approach showed acceptable results with levels of Def2-TZVPfor Cu and Def2-SVP for other elements. In general, all the computational results indicate that the cycloaddition reaction favors the formation of the observed 1,4-regioisomer (5-halo-triazoles) through a direct π-activation mechanism.
Graphical abstract










Similar content being viewed by others
References
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021
Duan X, Zheng N, Li M, Sun X, Lin Z, Qiu P, Song W (2021) Remote ether groups-directed regioselective and chemoselective cycloaddition of azides and alkynes. Chin Chem Lett 32(12):4019–4023
Kowalski K (2023) A brief survey on the application of metal-catalyzed azide–alkyne cycloaddition reactions to the synthesis of ferrocenyl-x-1, 2, 3-triazolyl-R (x = none or a linker and R= organic entity) compounds with anticancer activity. Coord Chem Rev 479:214996
Kumar V, Lal K, Tittal RK (2023) The fate of heterogeneous catalysis & click chemistry for 1, 2, 3-triazoles: nobel prize in chemistry 2022. Catal Commun 176:106629
Kalra P, Kaur R, Singh G, Singh H, Singh G, Kaur G, Singh J (2021) Metals as “click” catalysts for alkyne-azide cycloaddition reactions: an overview. J Organomet Chem 944:121846
Huisgen R (1989) Kinetics and reaction mechanisms: selected examples from the experience of forty years. Pure Appl Chem 61(4):613–628
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem 114(14):2708–2711
Tornøe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase:[1, 2, 3]-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67(9):3057–3064
Meldal M, Tornøe CW (2008) Cu-catalyzed azide− alkyne cycloaddition. Chem Rev 108(8):2952–3015
Hein JE, Fokin VV (2010) Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper (I) acetylides. Chem Soc Rev 39(4):1302–1315
Rasolofonjatovo E, Theeramunkong S, Bouriaud A, Kolodych S, Chaumontet M, Taran F (2013) Iridium-catalyzed cycloaddition of azides and 1-bromoalkynes at room temperature. Org Lett 15(18):4698–4701
Salam N, Sinha A, Roy AS, Mondal P, Jana NR, Islam SM (2014) Synthesis of silver–graphene nanocomposite and its catalytic application for the one-pot three-component coupling reaction and one-pot synthesis of 1, 4-disubstituted 1, 2, 3-triazoles in water. RSC Adv 4(20):10001–10012
Connell TU, Schieber C, Silvestri IP, White JM, Williams SJ, Donnelly PS (2014) Copper and silver complexes of tris (triazole) amine and tris (benzimidazole) amine ligands: evidence that catalysis of an azide-alkyne cycloaddition (“click”) reaction by a silver tris (triazole) amine complex arises from copper impurities. Inorg Chem 53(13):6503–6511
Boominathan M, Pugazhenthiran N, Nagaraj M, Muthusubramanian S, Murugesan S, Bhuvanesh N (2013) Nanoporous titania-supported gold nanoparticle-catalyzed green synthesis of 1, 2, 3-triazoles in aqueous medium. ACS Sustain Chem Eng 1(11):1405–1411
Smith CD, Greaney MF (2013) Zinc mediated azide–alkyne ligation to 1, 5-and 1, 4, 5-substituted 1, 2, 3-triazoles. Org Lett 15(18):4826–4829
Hong L, Lin W, Zhang F, Liu R, Zhou X (2013) Ln [N (SiMe 3) 2] 3-catalyzed cycloaddition of terminal alkynes to azides leading to 1, 5-disubstituted 1, 2, 3-triazoles: new mechanistic features. Chem Commun 49(49):5589–5591
Boren BC, Narayan S, Rasmussen LK, Zhang Li, Zhao H, Lin Z, Jia G, Fokin VV (2008) Ruthenium-catalyzed azide—alkyne cycloaddition: scope and mechanism. J Am Chem Soc 130(28):8923–8930
Egbert JD, Cazin CS, Nolan SP (2013) Copper N-heterocyclic carbene complexes in catalysis. Catal Sci Technol 3(4):912–926
Ahlquist M, Fokin VV (2007) Enhanced reactivity of dinuclear copper (I) acetylides in dipolar cycloadditions. Organometallics 26(18):4389–4391
Straub BF (2007) µ-Acetylide and µ-alkenylidene ligands in “click” triazole syntheses. Chem Commun 37:3868–3870
Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV (2005) Copper (I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc 127(1):210–216
Özen C, Tüzün NŞ (2012) The mechanism of copper-catalyzed azide–alkyne cycloaddition reaction: a quantum mechanical investigation. J Mol Graph Model 34:101–107
Cantillo D, Ávalos M, Babiano R, Cintas P, Jiménez JL, Palacios JC (2011) Assessing the whole range of CuAAC mechanisms by DFT calculations—on the intermediacy of copper acetylides. Org Biomol Chem 9(8):2952–2958
Calvo-Losada S, Pino MS, Quirante JJ (2014) On the regioselectivity of the mononuclear copper-catalyzed cycloaddition of azide and alkynes (CuAAC). A quantum chemical topological study. J Mol Model 20(4):1–7
Calvo-Losada S, Pino-González MS, Quirante JJ (2015) Rationalizing the catalytic activity of copper in the cycloaddition of azide and alkynes (CuAAC) with the topology of ∇ 2ρ (r) and ∇∇ 2ρ (r). J Phys Chem B 119(4):1243–1258
Straub BF, Bessel M, Berg R (2011) Dicopper catalysts for the azide alkyne cycloaddition: a mechanistic DFT study. Modeling of molecular properties. Wiley, New Jersey, pp 207–214
Wang C, Ikhlef D, Kahlal S, Saillard JY, Astruc D (2016) Metal-catalyzed azide-alkyne “click” reactions: Mechanistic overview and recent trends. Coord Chem Rev 316:1–20
Lahann J (ed) (2009) Click chemistry for biotechnology and materials science. Wiley, New Jersey
Kuijpers BH, Dijkmans GC, Groothuys S, Quaedflieg PJ, Blaauw RH, van Delft FL, Rutjes FP (2005) Copper (I)-mediated synthesis of trisubstituted 1, 2, 3-triazoles. Synlett 2005(20):3059–3062
Hein JE, Tripp JC, Krasnova LB, Sharpless KB, Fokin VV (2009) Copper (I)-catalyzed cycloaddition of organic azides and 1-iodoalkynes. Angew Chem 121(43):8162–8165
Lal S, Rzepa HS, Diez-Gonzalez S (2014) Catalytic and computational studies of N-heterocyclic carbene or phosphine-containing copper (I) complexes for the synthesis of 5-iodo-1, 2, 3-triazoles. ACS Catal 4(7):2274–2287
Badawi MAAH, Khairbek AA, Thomas R (2023) Computational studies of the CuAAC reaction mechanism with diimine and phosphorus ligands for the synthesis of 1, 4-disubstituted 1, 2, 3-triazoles. New J Chem 47(8):3683–3691
Khairbek AA, Badawi MAAH (2023) Mechanism of Ag (I)-catalyzed azide-alkyne cycloaddition reaction: a quantum mechanical investigation. React Kinet Mech Catal 136(1):69–81
Badawi M (2023) Mechanism of copper (I)-catalyzed cycloaddition of azides to terminal alkynes: a quantum mechanical investigation. Tishreen Univ J Basic Sci Ser 45(1):57–69
Badawi MAAH (2022) Mechanism of diethylamine/DBU-catalyzed cycloaddition of azides to unsaturated aldehydes: a quantum mechanical investigation. Comput Theor Chem 1209:113593
Al-Hakim Badawi MA, Al-Zaben MI, Thomas R (2023) DFT studies on mechanism of organocatalytic metal-free click 32CA reaction for synthesis of NH-1, 2, 3-triazoles. Catal Lett. https://doi.org/10.1007/s10562-023-04374-3
Rajimon KJ, Elangovan N, Khairbek AA, Thomas R (2023) Schiff bases from chlorine substituted anilines and salicylaldehyde: synthesis, characterization, fluorescence, thermal features, biological studies and electronic structure investigations. J Mol Liq 370:121055
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford CT, 2016, GaussView 5.0., Wallingford, E.U.A. https://gaussian.com/citation/
Peverati R, Truhlar DG (2012) An improved and broadly accurate local approximation to the exchange–correlation density functional: The MN12-L functional for electronic structure calculations in chemistry and physics. Phys Chem Chem Phys 14(38):13171–13174
Rappoport D, Furche F (2010) Property-optimized Gaussian basis sets for molecular response calculations. J Chem Phys 133(13):134105
Frau J, Glossman-Mitnik D (2018) Conceptual DFT study of the local chemical reactivity of the dilysyldipyrrolones A and B intermediate melanoidins. Theoret Chem Acc 137(5):1–10
Peintinger MF, Oliveira DV, Bredow T (2013) Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J Comput Chem 34(6):451–459
Sun Y, Hu L, Chen H (2015) Comparative assessment of DFT performances in Ru-and Rh-promoted σ-bond activations. J Chem Theory Comput 11(4):1428–1438
Silva PJ, Bernardo CE (2018) Influence of alkyne and azide substituents on the choice of the reaction mechanism of the Cu+-catalyzed addition of azides to iodoalkynes. J Phys Chem A 122(37):7497–7507
Bickelhaupt FM, Houk KN (2017) Analyzing reaction rates with the distortion/interaction-activation strain model. Angew Chem Int Ed 56(34):10070–10086
Oakdale JS, Sit RK, Fokin VV (2014) Ruthenium-catalyzed cycloadditions of 1-haloalkynes with nitrile oxides and organic azides: synthesis of 4-haloisoxazoles and 5-halotriazoles. Chem A Eur J 20(35):11101–11110
Arenas JL, Crousse B (2021) An overview of 4-and 5-halo-1, 2, 3-triazoles from cycloaddition reactions. Eur J Org Chem 2021(18):2665–2679
Li L, Li Y, Li R, Zhu A, Zhang G (2011) A new synthetic protocol for one-pot preparations of 5-halo-1, 4-disubstituted-1, 2, 3-triazoles. Aust J Chem 64(10):1383–1389
Wang B, Zhang J, Wang X, Liu N, Chen W, Hu Y (2013) Tandem reaction of 1-copper (I) alkynes for the synthesis of 1, 4, 5-trisubstituted 5-chloro-1, 2, 3-triazoles. J Org Chem 78(20):10519–10523
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khairbek, A.A., Alzahrani, A.Y., Badawi, M.A.AH. et al. Computational studies on CuAAC reaction mechanism with [CuX(PPh3)]; X = I, Br, Cl for the synthesis of 4- and 5-halo-1,2,3-triazoles. Reac Kinet Mech Cat 137, 777–790 (2024). https://doi.org/10.1007/s11144-023-02548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02548-z