Abstract
The substitution behaviour of bidentate N,N′-donor (pyridin-2-yl)methyl-aniline chelates with different substituents on the mononuclear Pd(II) complexes was investigated. The complexes were synthesized and characterized using 1H NMR, 13C NMR, FTIR, LC–MS, and elemental analysis. The study explored the kinetics and mechanistic behaviour of N,N′-pyridyl Pd(II) complexes, viz. dichloro-(N-((pyridin-2-yl)methyl)aniline)palladium(II) (PdL1), dichloro-(4-fluoro-N-((pyridin-2-yl)methyl)aniline)-palladium(II) (PdL2), dichloro-(4-methoxy-N-((pyridin-2-yl)methyl)aniline)-palladium(II) (PdL3) and dichloro-(4-ethyl-N-((pyridin-2-yl)methyl)aniline)-palladium(II) (PdL4). The effect of concentration and temperature on substitution behaviour of coordinated chloride ligands by three bio-relevant thiourea nucleophiles, viz. thiourea (TU), N,N′-dimethylthiourea (DMTU) and N,N,N′,N′-tetramethylthiourea (TMTU), of different steric demands was studied in a 0.1 M (LiCl) solution of ultra-pure water under pseudo-first order conditions using standard Stopped-Flow and UV–Visible spectrophotometric techniques. The substitution of the chloride ligands from the Pd(II) metal by thiourea nucleophiles was a two-step reaction, where the chloride trans to the pyridine ligand was substituted first due to the strong trans effect on the pyridine ring compared to the amine group. The rate of substitution of the chloride by thiourea nucleophiles increased with the presence of an electron-withdrawing substituent and decreased when an electron-donating group was attached to the para position of the phenyl moiety. The negative activation entropies and positive activation enthalpy for all the substitution reactions support an associative mode of substitution mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal-based anticancer agents have become a common strategy in cancer therapy, owing the attention to the discovery of cisplatin and other common platinum-based derivatives [1,2,3]. The design of novel metallodrugs results from the side effects associated with platinum(II) drugs, such as neurotoxicity, renal toxicity, hair loss, nausea, and vomiting [4, 5]. The most severe side effects are linked to the lack of selectivity of the drugs, interactions with thiol groups of proteins, and acquired resistance; consequently, efficacy is compromised for clinical use [2, 4, 6, 7]. Due to similar coordination geometries and equilibrium behavior between platinum(II) and palladium(II) ions, Pd(II) complexes have emerged as prospective chemotherapeutic agents and have displayed promising cytotoxicity effects against cisplatin-resistant cells [8,9,10].
Despite this, the application of palladium-based therapeutics is compromised by drawbacks such as rapid aquation and high rates of ligand exchange in solution [11]. The lability of Pd(II) complexes is 105 fold more than their Pt(II) analogues. Such high reactivities suggests that these complexes are ideal models for kinetic and mechanistic studies in the presence of sulfur and nitrogen donor biomolecules [7]. The quick ligand exchange of Pd(II) analogues in vivo is associated with the Pd(II) ion’s strong affinity for soft sulfur and nitrogen-containing biomolecules [7, 12]. In an effort to enhance interactions of Pd(II) complexes with the target DNA molecule, Pd(II) complexes with controlled rates constants have been investigated [13,14,15]. The ligand environment around the metal center has been shown to significantly influence the stability and, hence, the reactivity of Pd(II) complexes [16]. Substantial studies have been reported on the modification of palladium-based complexes through careful manipulation of the coordinating ligand σ-donor/π-acceptor capacities, insertion of bulky aliphatic or aromatic nitrogen ligands, and even the design of multinuclear Pd(II) complexes [17,18,19,20,21]. The electronic and steric effects of such chelate ligands can regulate the rate of substitution and thermodynamic properties of Pd(II) complexes thus improving the cytotoxic effect against cancer cells through kinetic deceleration effect [13, 22, 23].
Ch Moi and co-workers evaluated the substitution behavior of [Pd(Ambim)(H2O)2]X2, where Ambim is 2-(aminomethyl)benzimidazole, a bidentate non-leaving benzimidazole ligand [15]. They studied the kinetic parameters of the reactivity of the diaqua complex towards selected sulphur-containing biomolecules. The kinetic measurements showed that the nucleophiles reacted with the complex via two successive steps following an associative substitution mode. The study indicated that the pseudo first order rate constant for the first substitution step (kobs(1st)) was depended on nucleophile concentration while the pseudo first order rate constant for the second substitution step (kobs(2nd)) was independent of the nucleophile concentration. The rate constant for the second substitution step was found to be 10−3 s−1 and is attributed to a decrease in the electrophilicity of the Pd(II) centre along with an increase in steric hindrance around it, arising from the incorporation of the first nucleophile.
Pyridylmethyl-amines are common bidentate ligands that have been coordinated to several transition metals due to their versatile structure, which can be modified by substitution on the amine group or the pyridyl units [24, 25]. The polydentate characteristics of these ligands allow for a variation of steric and electronic properties, resulting in metal complexes with diverse chemical applications [26, 27]. Despite the abundant reports on pyridylmethyl-amine and analogues of transition metal complexes, there is not much information on the substitution behavior of the labile group in palladium(II) complexes coordinated with pyridylmethyl-amine ligands.
Given the diverse applications of pyridylmethyl-amine ligands, N,N′-bidentate pyridylmethyl-amine based Pd(II) complexes with systematically varied substituents on the para position of the phenyl group were synthesized. We explored the role of structural and electronic properties of the coordinate ligands on the substitution behaviour of labile chloride ligands by thiourea nucleophiles. The thiourea nucleophiles, viz. thiourea (TU), N,N′-dimethylthiourea (DMTU) and N,N,N′,N′-tetramethylthiourea (TMTU), which are highly soluble and nucleophilic, were selected in this study to mimic the biological role played by sulfur-containing molecules in the human blood [28]. DFT calculations such as geometry optimisation and molecular orbital energy and NBO analyses were performed to investigate the electronic properties of the complexes.
Experimental section
Materials and methods
The reagents anilines, sodium triacetoxyborohydride (STAB), sodium hydrogen carbonate, magnesium sulfate, silica gel, and palladium(II) chloride were purchased from Sigma-Aldrich in analytical grade. The precursor [Pd(CH3CN)2Cl2] was synthesized from the reflux reaction of PdCl2 and acetonitrile. All synthesis of ligands and Pd(II) complexes were performed under an inert nitrogen atmosphere. The solvents hexane and dichloromethane were purchased from Sigma-Aldrich and dried following appropriate methods: hexane was dried through standard distillation methods, while dichloromethane was stored in activated 3 Å molecular sieves. All other solvents were purchased from Sigma-Aldrich and were used without further purification.
Physical measurements and instrumentation
1H and 13C NMR spectra were acquired on Bruker Avance III 500 MHz and 100 MHz spectrometers with a 5 mm TBIZ probe at 30 °C. Chemical shifts were recorded in ppm relative to the solvent residual peak, CDCl3, and DMSO-d6 for ligands and complexes. The supplementary information shows the exemplary 1H and 13C NMR spectra of the ligands and the complexes. FT-IR spectral data were acquired using a Bruker Alpha II FT-IR spectrometer, and the data was recorded as percentage transmittance at the wavenumber (cm−1) within the range 500–4000 cm−1. Low-resolution mass spectral data was collected on a Waters TOF Micro-mass LCT Premier spectrometer for the synthesized complexes. Elemental compositions of the complexes were determined using CHNS Thermo Scientific Flash 2000 analyzer. An Applied Photophysics SX 20 Stopped-Flow spectrophotometer and a Cary 3500 UV–Vis spectrophotometer coupled with an online acquisition system were used to study the substitution reactions of the Pd(II) complexes.
Density functional theoretical calculations
Gaussian 09 program suite [29] was used to optimize the theoretical ground-state structures of PdL1–PdL4 in the gas phase using B3LYP (Becke 3-Lee-Yang-Parr) functional mode in combination with 6-31G (C and H), 6-311+G (N, S, and Cl), and (Los Alamos National Laboratory 2 double ζ) LANL2DZ (Pd)) basis sets [30, 31]. This method is suitable for gaseous simulations of compounds that contain heavy transition metal atoms such as Pd. Natural bond orbitals (NBO) analysis was used to calculate atomic charges in the complexes. Quantum chemical descriptors, such as dipole moment, electrophilicity indices, chemical potential and chemical hardness for the optimized complexes were determined.
Synthesis of ligands
The ligands (L1–L4) were synthesized according to a modified method by Mundinger et. al [32]. The para-substituted anilines (1 mmol) and 2-pyridine-carboxaldehyde (0.0951 ml, 1 mmol) were dissolved in dry dichloromethane (10 ml), and then solid STAB (0.3179 g, 1.5 mmol) was added to the reaction mixture. The reaction mixture was stirred at room temperature under nitrogen gas for 6 h, and the progress of the reaction was monitored by TLC using Hexane/Ethyl acetate 7:3. The resulting reaction mixture was quenched with saturated sodium hydrogen carbonate solution, which was then extracted three times with dichloromethane. The combined organic phase layer was dried over magnesium sulfate, and the solvent was removed under reduced pressure to obtain an oily liquid. The crude product was purified using column chromatography (hexane/ethyl acetate 7:3). The isolated desired ligands are described hereafter.
N-(pyridin-2-ylmethyl) aniline
L1 was obtained as a yellow oil (95.6 mg, 51.89%). 1H NMR (400 MHz, CDCl3, ppm): 8.60 (d, 1H, J = 4.81 Hz), 7.65 (t, 1H, J = 7.65 Hz), 7.36 (d, 1H, J = 7.92 Hz), 7.21 (m, 3H), 6.77–6.69 (m), 4.47 (s, 2H), 4.44 (s, 1H). 13C NMR (CDCl3, 400 MHz): 158.58, 149.09, 147.93, 136.76, 129.27, 122.14, 121.66, 117.61, 113.09, 49.26. FT-IR (liquid neat; cm−1) 3386.90 (–NH), 1597.51 (C=C), 1264.76 (C–N aromatic).
4-Fluoro-N-(pyridin-2-ylmethyl) aniline
L2 was obtained as an orange oil (127.9 mg, 63.24%). 1H NMR (400 MHz, CDCl3, ppm): 8.58 (d, 1H), 7.62 (t, 1H), 7.31 (d, 1H), 7.17 (t, 1H), 6.88 (t, 2H), 6.60–6.57 (m, 2H), 4.54 (s, 1H), 4.40 (s, 2H). 13C NMR (CDCl3, 100 MHz): 158.32, 149.16, 144.36, 144.35, 136.69, 122.18, 121.66, 115.74, 115.52, 113.86, 113.79, 49.81. FT-IR (liquid neat; cm−1) 3264.34 (–NH), 1501.16 (C=C), 1301.22 (C–N aromatic), 1203.19 (C–F).
4-Methoxy-N-(pyridin-2-ylmethyl) aniline
L3 was obtained as a dark orange oil (96.6 mg, 45.08%). 1H NMR (400 MHz, CDCl3, ppm): 8.60 (d, 1H), 7.67 (t, 1H), 7.38 (d, 1H), 7.20 (t, 1H), 6.80–6.77 (m, 2H), 6.67–6.64 (m, 2H), 4.44 (s, 2H), 4.09 (s, 1H), 3.75 (s, 3H). 13C NMR (CDCl3, 100 MHz): 158.77, 152.36, 148.95, 141.98, 136.90, 122.17, 121.83, 114.93, 114.45, 55.78, 50.20. FT-IR (liquid neat; cm−1) 3277.26 (–NH), 1502.66 (C=C), 1290.3 (C–N aromatic), 1225.65 (C–O).
4-Ethyl-N-(pyridin-2-ylmethyl) aniline
L4 was obtained as a dark orange oil (102 mg, 48.04%). 1H NMR (400 MHz, CDCl3, ppm): 8.62 (d, 1H), 7.63 (t, 1H), 7.37 (d, 1H), 7.18 (t, 1H), 7.08 (d, 2H), 6.68 (d, 2H), 4.48 (s, 2H), 4.39 (s, 1H), 2.61 (m, 2H), 1.25 (t, 3H), 13C NMR (CDCl3, 100 MHz): 158.98, 149.16, 145.98, 136.69, 133.40, 128.62, 122.07, 121.64, 113.24, 49.69, 27.99, 15.99. FT-IR (liquid neat; cm−1) 3385.35 (–NH), 2947.94 (C–H alkane), 1512.12 (C=C), 1261.96 (C–N aromatic).
Synthesis of corresponding Pd(II) complexes
The corresponding Pd(II) complexes (PdL1–PdL4) were synthesized according to a procedure reported by Kim and co-workers [27].
Dichloro-(N-((pyridin-2-yl)methyl)aniline)-palladium(II), PdL1
A solution of L1 (40 mg, 0.22 mmol) in dry DCM (10 ml) was added to a solution of [Pd(CH3CN)2Cl2] (57.07 mg, 0.22 mmol) in 5 ml of dry DCM. The reaction mixture was stirred overnight at room temperature under nitrogen gas. The solvent was reduced by a rotary evaporator, the resulting solid residue was filtered and washed with cold ethanol (10 ml × 3), to obtain a yellow solid (64.85 mg, 81.53%). Mp: 246.3–249.6 °C. 1H NMR (500 MHz DMSO-d6, ppm): 8.76 (m, 2H), 8.18 (t, 1H), 7.79 (d, 1H), 7.60 (t, 1H), 7.32 (t, 2H), 7.23(t, 1H) 7.12 (d, 2H), 5.01–4.95 (m, 1H), 4.41 (d, 1H). 13C NMR (100 MHz, DMSO-d6, ppm): 164.33, 149.40, 146.85, 141.00, 129.81, 126.40, 124.79, 122.53, 121.46, 61.29. FT-IR (liquid neat; cm−1) 3372.10 (–NH), 1649.06 (C=N). TOF-MS ES+: m/z = 384.94 (calculated m/z 361.56), [(M + Na)+]. Anal. % Calculated for C12H12Cl2N2Pd: C, 39.86; H, 3.35; N, 7.75. Found (%): C, 40.03; H, 3.31; N, 7.66.
Dichloro-(4-fluoro-N-((pyridin-2-yl)methyl)aniline)-palladium(II), PdL2
The complex PdL2 was prepared according to a similar procedure as PdL1 except for the use of L2 (47 mg, 0.2 mmol). PdL2 was obtained as a yellow solid (68.7 mg, 90.50%). Mp: 244.5–247.2 °C. 1H NMR (500 MHz, DMSO-d6, ppm): 8.84 (s, broad, 1H) 8.77 (d, 1H), 8.17 (t, 1H), 7.77 (d, 1H), 7.60 (t, 1H), 7.18 (t, 4H), 4.95–4.90 (dd, 1H), 4.46–4.43 (dd, 1H). 13C NMR (100 MHz, DMSO-d6, ppm): 163.89, 149.43, 143.18, 140.99, 124.83, 123.60, 123.53, 122.60, 116.36, 61.56. FT-IR (liquid neat; cm−1) 3374.34 (–NH), 1652.33 (C=N), 992.91 (C–F). TOF–MS ES+, m/z = 422.98 (calculated m/z 379.55), [M+–Cl + DMSO]. Anal. % Calculated for C12H11FCl2N2Pd: C, 37.97; H, 2.92; N, 7.38. Found (%): C, 37.69; H, 2.97; N, 7.22.
Dichloro-(4-methoxy-N-((pyridin-2-yl)methyl)aniline)-palladium(II), PdL3
The complex PdL3 was prepared according to a similar procedure as PdL1 except for the use of L3 (26.7 mg, 0.12 mmol). PdL3 was obtained as a yellow solid (37.3 mg, 79.38%). Mp: 214.2–216.8 °C. 1H NMR (400 MHz DMSO-d6, ppm): 8.78 (s, 1H) 8.66 (s, 1H), 8.17 (t, 1H), 7.78 (d, 1H), 7.60 (t, 1H), 7.08 (d, 2H), 6.89 (d, 2H) 4.96–4.91 (m, 1H), 4.35 (d, 1H),3.72 (s, 3H). 13C NMR (100 MHz, DMSO-d6, ppm): 168.06, 156.63, 152.12, 146.24, 139.61, 125.26, 123.08, 117.32, 117.02, 59.88, 56.26. FT-IR (liquid neat; cm−1) 3376.48 (–NH), 1652.38 (C=N), 993.18 (C–O). MS ES+, m/z = 414.61 (calculated m/z 391.59), [(M + Na)+]. Anal.% Calculated for C13H14Cl2N2OPd: C, 39.87; H, 3.60; N, 7.16. Found (%): C, 39.98; H, 3.68; N, 7.21.
Dichloro-(4-ethyl-N-((pyridin-2-yl)methyl)aniline)-palladium(II), PdL4
The complex PdL4 was prepared according to a similar procedure as PdL1 except for the use of L4 (25.46 mg, 0.12 mmol). PdL4 was obtained as a yellow solid (27.2 mg, 58.18%). Mp: 187.3–190.2 °C. 1H NMR (500 MHz DMSO-d6, ppm): 8.76 (d, 1H), 8.70 (s, 1H),8.18 (t, 1H), 7.78 (d, 1H), 7.60 (t, 1H), 7.14 (d, 2H), 7.03 (d, 2H), 5.00–4.94 (m, 2H), 4.36 (d, 1H), 2.51 (m, 2H), 1.15 (t, 3H). 13C NMR (100 MHz, DMSO-d6, ppm): 164.38, 149.39, 144.59, 142.04, 140.95, 128.63, 124.74, 122.54, 113.09, 61.50, 28.05, 16.47. FT-IR (liquid neat; cm−1) 3372.91 (–NH), 1651.42 (C=N). TOF–MS ES+, m/z = 433.04 (calculated m/z 389.62), [M+–Cl + DMSO]. Anal. % Calculated for C14H16Cl2N2Pd: C, 43.16; H, 4.14; N, 7.19. Found (%): C, 43.45; H, 4.01; N, 7.25.
Kinetic measurements
Stock solutions of Pd(II) complexes and solutions of nucleophiles were prepared by dissolving known amounts of each in ultra-pure water with an ionic strength of 0.1 M (LiCl). Lithium chloride was added to prevent spontaneous solvolysis of the Pd(II) complexes. The concentrations of the Pd(II) complexes were maintained at 0.05 mM, while the solutions of TU and DMTU were prepared at concentrations of 10, 20, 30, 40, and 50-fold in excess. TMTU solutions were prepared to afford concentrations of 20, 40, 60, 80, and 100-fold more than that of the metal complex. This was due to an observed slow reactivity of the TMTU nucleophile compared to TU and DMTU. The subsequent dilutions of nucleophile solutions ensured pseudo first-order conditions. Equal volumes of complexes and nucleophiles were mixed and administered on the UV–Visible and Stopped-Flow spectrophotometers. The spectral changes arising from the reactions of Pd complexes and nucleophiles were monitored from 800 to 200 nm, to determine suitable wavelengths for the kinetic measurements. The observed pseudo first order rate constant, kobs was calculated as the average value from five independent kinetic runs on the Stopped Flow and as duplicate runs from the UV–Visible experiments.
Results
Synthesis of Pd(II) complexes
The condensation reaction of 2-pyridinecarboxaldehyde and the corresponding para substituted anilines in the presence of STAB reagent afforded N,N-(pyridin-2-yl)methyl-aniline ligands in moderate yields (45–63%) (Fig. 1). Treatment of ligands with bis(acetonitrile)palladium dichloride [PdCl2(CH3CN)2] produced the corresponding Pd(II) complexes in good to excellent yields (58–90%). Synthesis of the ligands and their corresponding Pd(II) complexes were confirmed by NMR, FT-IR spectroscopy, melting point, TOF-Mass Spectrometry and elemental analysis.
The 1H NMR spectra of ligands and corresponding Pd(II) complexes show expected multiplicities and integrations. In general, a signature peak at 4.41–4.51 ppm was observed for all synthesized ligands. This singlet signal was assigned to the protons of the diastereotopic methylene group of the ligand, this validates the reduction of the aldehyde carbonyl (C=O) by the STAB reagent and hence the coupling of the pyridyl moiety to the aniline. Additionally, the amine hydrogen atom resonates at 3.95–4.55 ppm as a broad (–NH) peak observed as a singlet adjacent to the region of the methylene group protons for all synthesized ligands. In contrast, while the diastereotopic methylene group protons were observed as a singlet on the free ligand 1H NMR spectra, a well-resolved ABX spin system for the protons of the methylene group of the chelating ring (HA/B and HB/A) and the amine proton (HX) was observed for all complexes. To further elucidate the coupling between the diastereotopic methylene group protons and the amine proton, COSY spectra for the complexes was obtained, Fig. S5 (ESI) shows an exemplary COSY spectrum for PdL1. A diagnostic change was observed for the amine proton (–NH) through a significant shift from the 4.0–4.5 ppm region to about 8.8 ppm when the ligands were complexed to the Pd metal. An overall notable downfield shift was observed for all the aromatic protons (1H NMR) and carbons (13C NMR) of the complexes compared to the free ligands, thus confirming that the Pd is attached to the ligand. The identity of Pd(II) complexes was further confirmed by comparing their FT-IR spectra to their corresponding ligands. The spectra of all complexes indicate the amine stretch as broader and more pronounced than in the ligands, which indicates a change in the chemical properties of the –NH group and confirms coordination of the ligand to the palladium metal atom. Elemental analysis data of the complexes were consistent with the proposed Pd(II) structures in Fig. 1 and confirmed the purity and stoichiometric ratios of the complexes.
DFT–computational modelling and analysis
Computational simulations and optimized DFT calculations were performed to gain insight on the electronic and structural properties of the synthesized Pd(II) complexes. The data obtained was used to explain the reactivity of the synthesized complexes.
The optimized geometry of frontier molecular orbitals (HOMO and LUMO) and planarity of the complexes are presented in Table 1, while the key DFT data of the optimized complexes are summarized in Table 2. All complexes adopt a distorted square planar geometry in which the Pd–N1 and Pd–N2 bond lengths are not equal.
The frontier orbitals of all complexes have similar features due to the similar basic structures. The DFT optimized structures in Table 1 reveal that the electron densities of the highest occupied molecular orbital (HOMO) are predominantly localized on the Pd(II) metal center and the chloride ligands; while the lowest unoccupied molecular orbitals (LUMO) are distributed along the chlorides, palladium center, and coordinate bidentate ligand. Also presented in Table 1, are DFT optimized planarity structures showing a similar planarity of all the complexes, with the pyridyl moiety and chloride ligands in-plane with the metal center, while the para substituted aniline moiety is noticeably out-of-plane for all the complexes. This is due to the flexibility brought by the methylene group within the inert ligand. The computational data is comparable with that reported in literature for the crystal structures of PdL1, PdL2 and PdL3 [27, 33,34,35,36].
Based on the computational data presented in Table 2, the introduction of alternate electron withdrawing and electron donating substituents on the inert ligand have little to no significant effect on the Pd–Cl bond length, bond angle or NBO charges of the palladium metal. However, a notable trend is observed in the overall electrophilicity of the complexes; with an increase in electrophilicity in the presence of an electron withdrawing substituent, i.e., F (5.5968), and reduced electrophilicity for an electron donating group, CH2CH3 (5.0711) when using PdL1 (5.3180) as a reference. The dipole moment of the complex with an electron withdrawing fluoro group, PdL2 is lower than that of PdL1, the unsubstituted aniline complex, while the PdL4, with electron donating group (–CH2CH3) has the highest dipole moment (13.9099). Similarly, structural modification on the complexes almost has no impact on the composition of the frontier orbitals, however, the trend in the computed energy gap, ∆ELUMO–HOMO noticeably increases in the order PdL2 < PdL1 < PdL4 < PdL3. This trend indicates an increase of electron donation density around the Pd(II) metal center which is supported by trend of HOMO and LUMO energy levels.
Ligand substitution kinetics and activation parameters
Substitution of labile chloride ligands from the Pd(II) complexes by three thiourea nucleophiles, viz. thiourea (TU), N,N′-dimethylthiourea (DMTU) and N,N,N′,N′-tetramethylthiourea (TMTU) was studied under pseudo-first order conditions, as a function of concentration and temperature using the UV–Vis and stopped-flow spectrophotometer. The selected wavelengths are presented in Table S1. The substitution of the four dichloro complexes with the thiourea nucleophiles occurred in two consecutive steps as shown in Fig. 2. Each step represents the substitution of one of the chloride ligands, due to the different trans effect of the pyridine ring and the amine groups. The second step is the slower step, where another chloride ligand is substituted.
Substitution of the first chloride
The substitution of the first chloride ligand was monitored by the stopped flow technique. All kinetic traces obtained from concentration and temperature dependence analysis gave excellent fits to the single-exponential decay function. A typical kinetic trace generated from the Stopped-Flow spectrophotometer is shown in Fig. 3 for the reaction between PdL1 and TU nucleophile. Using an online Pro-Data SX programme, the kinetic traces were fitted into a non-linear least square fit to generate the observed pseudo first-order rate constant, kobs(1st) (Eq. 1) at specific nucleophile concentration. All the reported rate constants represent an average of five independent kinetic runs for each experimental condition.
where At is the absorbance at time t, Αο is the absorbance of reaction mixture initially and Α∞ is the absorbance at the end of the reaction.
The plots of average kobs(1st) against nucleophile concentrations, [Nu], afforded a linear regression with zero intercepts for all complexes, according to the rate law described by Eq. 2. A representative plot of kobs versus concentration of all three nucleophiles for PdL3 is shown in Fig. 4. The kobs values for the first substitution step are found in Tables S2–S5 and representative plots of kobs against concentration are indicated by Figs. S22–S25 in the Electronic Supplementary Information. All linear regression plots pass through zero, suggesting that all the reactions are irreversible. The second order rate constants (k1), calculated from the slopes of kobs(1st) against nucleophile concentration are summarised in Table 3.
Substitution of the second chloride
The second substitution step was monitored on the UV–Visible spectrophotometer and is the substitution of the second chloride ligand by the second thiourea nucleophile. The kinetic traces of the ligand substitution reactions gave excellent fits to a single-exponential decay function. The values of kobs(2nd) considered in subsequent analysis are duplicate runs from the UV–Visible experiments for each experimental condition. Fig. 5 shows a representative UV–Visible spectrum observed for the reaction of PdL1 with DMTU and the corresponding absorbance vs time trace, followed at 300 nm.
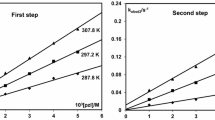
The second substitution step is slow for all complexes and is independent of changes in nucleophile concentration. Fig. 6 indicates the effect of concentration change on the first and second substitution steps for PdL1 and TU. The kobs(2nd) values at varied concentration of the nucleophiles are found in Tables S9–S12. The observed rate constants, kobs(2nd) values were considered to be equivalent to the rate constant for the second substitution step, k2, [37,38,39,40] and are indicated in Table 3 along with the values of k1.
Effect of temperature on the reaction rate
Temperature dependence reactions of k1 and k2 were monitored over the temperature range of 288–308 K, at 5 K intervals. The rate constant for both the substitution steps (k1 and k2) were found to increase with increasing temperatures over the studied temperature ranges. The enthalpy of activation (\(\Delta {H}^{\ne }\)) and entropy of activation (\(\Delta {S}^{\ne }\)) were calculated from the Eyring plots (Figs. 7 and 8) using Eq. 3. \(\ln\frac{{k}_{1}}{T}\) and their \(\frac{1}{T}\) values are represented by Tables S6–S8 for the displacement of the first chloride and their Eyring plots are in Figs. S26–S28. Tables S13–S15 show the displacement of the second chloride and their Eyring plots are indicated by Figs. S29–S30. The enthalpy of activation (\(\Delta {H}^{\ne }\)) and entropy of activation (\(\Delta {S}^{\ne }\)) are summarized in Table 4. The calculated activation parameters for all reactions corresponded well with those found for similar square planar d8 complexes in the literature [15, 37, 40, 41].
1H NMR Spectroscopy
Two consecutive substitution steps were observed for all studied complexes. It is therefore important to understand whether the two substitution steps were due to successive substitution of the chloride by thiourea or simultaneous substitution of the chloride groups followed by dechelation of the ligand from Pd(II) metal. Since the Pd(II) complexes used in the study comprise of two types of nitrogen bonded to the Pd metal, i.e. the aromatic pyridine and the sp3-hybridized primary amine, the trans effect of the pyridine ring nitrogen makes the chloride ligand trans to it more labile, which gets substituted first. The remaining chloride ligand trans to the primary amine group was replaced next by the second thiourea nucleophile. This difference in trans effect is strongly supported by the trends in the DFT data in Table 2. The NBO charges of N1(py) and N2(amine) or the bond lengths Pd–N1(py) and Pd–N2(amine) are significantly different. A similar trend was observed for Pt(II) complexes with similar ligands [37, 38, 42,43,44,45]. Fig. 2 shows the proposed stepwise substitution reaction of the chloride from the Pd metal by thiourea nucleophiles.
To confirm that the first substitution step is due to the displacement of one chloride ligand by the thiourea nucleophile while the second substitution step is assigned to the substitution of the second chloride ligand as shown in Fig. 2, the product of the substitution reaction of PdL1 (2.766 mM) by six equivalents of TU was monitored using 1H NMR spectroscopy. Fig. 9 shows the 1H NMR overlay spectra of the ligand (L1), PdL1 and the product of the reaction between PdL1 and TU. Due to the fast rate of substitution for the Pd(II) complex at high concentration, the substitution could not be monitored over time but instead, the final product that formed after the reaction was complete was analyzed. The numbering scheme employed for the protons in PdL1 is shown in the figure.
When observing the product of PdL1 and TU reaction; as diagnostic peaks, the methylene protons at 4.46 ppm resonate as a doublet with a coupling constant of 6.32 Hz, whilst in PdL1 the protons resonate as two distinct signals at 4.98 and 4.40 ppm. Additionally, the NH proton appears as a broad singlet at 6.26 ppm, compared to 4.27 ppm for the free ligand. This downfield shift indicates that the Pd atom is still coordinated to the amine.
Furthermore, the pyridyl proton and phenyl protons labelled Ha and Hi, were used to monitor the progress of the reaction, since they are closest to the N-donor atoms coordinated to the Pd(II) metal centre at which substitution occurs. Ha resonates at 8.60 ppm for the ligand, then shifts to 8.76 ppm upon complexation in PdL1 and an upfield shift to 8.58 ppm is observed when the chloride groups are displaced by TU. Similarly, Hi resonates at 2.69 ppm for the ligand, then shifts to 7.23 ppm for the unreacted PdL1 complex. Upon substitution by TU, Hi resonates at 6.58 ppm. Upon coordination of TU to the PdL1, Ha and Hi peaks are shifted upfield, due to replacement of the more electronegative chloride ligand by the TU.
Discussion
Four bidentate Pd(II) complexes comprising (pyridin-2-yl)methyl-aniline inert chelate ligands were synthesized and characterized. The complexes have substituents of varying electronic effects on the para-substituted N-((pyridin-2-yl)methyl)aniline ligand, i.e. –H, –CH3, –OCH3 and –CH3CH3. The molecular structures of these complexes show similarity in terms of planarity, bond lengths, and angles. The coordination geometry of the Pd(II) centre in all the complexes is slightly distorted square planar [36]. The para-substituted aniline moiety lies out-of-plane with respect to the rest of the pyridyl moiety of the ligand, Pd metal, and the two chlorides.
The chloride ligand substitution rate from these complexes by three thiourea nucleophiles viz. TU, DMTU, and TMTU was measured at different nucleophile concentrations and temperatures under pseudo-first-order conditions. Substitution of the two chloride ligands by the sulfur donor nucleophiles from the Pd(II) complexes took place via two consecutive reaction steps, Fig. 2. This is expected considering they are coordinated trans to two asymmetric donor sites of the N-((pyridin-2-yl)methyl)aniline chelate ligand. The first substitution step is concentration dependent and corresponds to the displacement of chloride trans to the pyridine ligand, due to the stronger trans effect of the pyridine ring compared to the amine group. This observation is supported by the bond lengths of Pd–Cl and the natural charges of nitrogen and chloride ligands (Table 2).
The second substitution step is the displacement of the second chloride ligand trans to the amine group by the nucleophiles. This step is slower compared to the first substitution step due to the coordinated thiourea nucleophile, which causes steric effects around the metal center and makes the Pd(II) metal center less electrophilic due to the donation of electron density towards the Pd(II) metal center. The second substitution step for all complexes is independent of concentration. The observed first-order rate constant for the displacement of the second chloro ligand by the thiourea nucleophile does not increase significantly when the concentration of the nucleophile is increased (Fig. 6). Even though the observed rate constant for the second substitution step was not influenced by changes in concentration of the nucleophile, it increased with an increase in temperature. The observed rate constants, kobs(2nd) values were considered to be equivalent to the rate constant for the second substitution step, k2 [37,38,39,40].
The product of the substituted PdL1 by thiourea (TU) nucleophile was analyzed by 1H NMR (Fig. 9). The NH proton of PdL1 appears as a broad singlet at 6.26 ppm, compared to 4.27 ppm for the ligand. The downfield shift confirms that the Pd atom is still coordinated to the NH. Similarly, the proton adjacent to the nitrogen of the pyridine ring (Ha) resonates at 8.76 ppm for PdL1, and at 8.58 ppm when the chloride groups are displaced by TU on PdL1, which further indicates that the pyridine moiety of the non-labile ligand is still coordinated to the Pd metal.
Using the second order rate constant (k1) values from the chloride substitutions as representative of reactivity for the first step (Table 3), the rate of reactivity followed the order PdL3 < PdL4 < PdL1 < PdL2 for all nucleophiles. The trend in reactivity is attributed to the difference in electronic effects within the complexes, which is supported by the electrophilicity index, chemical potential, chemical hardness, and the ∆ELUMO–HOMO, Table 2.
The higher reactivity of PdL2 is due to the electron density withdrawal from the ligand’s aniline moiety by the electronegative –F substituent, which pulls electrons from the electron-deficient amine coordinated to the metal center. This results in the loss of electron density from the ligand moiety and increases the electrophilicity of the metal center, as supported by the higher electrophilicity index (5.5967), leading to increased substitution. However, the presence of –F as an ancillary substituent on the aniline group does not significantly change electronic effects (bond lengths, bond angles or natural charges) when compared to PdL1 in Table 2.
In the case of PdL4, with electron donating group, –CH2CH3 on the para position of the aniline moiety, the reactivity is lower than that of PdL1, the unsubstituted complex and PdL2, complex with an electron-withdrawing substituent. The –CH2CH3 donates electrons to the phenyl ring and thus increase the electron density on the ring through the inductive donating effect. This reduces the electrophilicity of the metal centre for PdL4 (ω = 5.0711), thus reducing the reactivity compared to the unsubstituted PdL1.
PdL3 with the –OCH3 substituent has the lowest reactivity compared to all the studied Pd complexes. The positive σ-inductive effect of the methoxy substituent increases electron density of the phenyl ring and hence the acidic amine coordinated to the metal. This reduces the electrophilicity of the metal centers, as supported by the lower electrophilicity index (ω) of PdL3 (5.0144) compared to that of PdL1 (5.3180).
The reactivity of the second substitution step occurs trans to the amine group and involves the displacement of the second chloride ligand. The substitution step is slower compared to the first one, since the thiourea ligand has already entered the coordination sphere, thus increasing the electron density on the Pd(II) centre with its σ donating ability, which results in a slower nucleophilic attack. The reactivity of the second step follows the same order as the first step for the complexes and is influenced by electronic effects of the chelate ligand.
The general trend for substituting chloride ligands by thiourea nucleophiles for both the substitution steps follows the order TMTU < DMTU < TU. This order is compatible with the steric demands of the different nucleophiles, with the bulkier TMTU reacting slower than the less sterically hindered TU.
Based on the results tabulated in Table 4, the values of enthalpy of activation are positive, while the entropy of activation values are largely negative suggesting a compact transition state than that present in the starting reactants [15, 40]. This trend in thermodynamic parameters supports an associative mode of activation for the substitution step, which is in line with the square-planar d8 metal complexes [46].
Conclusion
Pd(II) complexes containing different para-substituted N-((pyridin-2-yl)methyl)aniline chelating spectator ligands of different electronic properties were synthesized and characterized by various spectroscopic methods. The kinetics and mechanism of their substitution reactions with sulfur-donor nucleophiles were studied under pseudo first-order conditions. The substitution of two chloride ligands by the nucleophiles from the Pd(II) complexes was consecutive. The chloride trans to the pyridine ligand was substituted first, since the pyridine has a stronger trans effect compared to the amine group. The rate of consecutive chloride substitution from the complexes by the nucleophiles followed the order PdL3 < PdL4 < PdL1 < PdL2. The higher reactivity of PdL2 is due to the withdrawal of electron density from the aniline moiety of the ligand by the –F substituent, which in turn pulls electrons from the electron deficient amine that is coordinated to the metal center. This results in the loss of electron density from the ligand moiety and increases the electrophilicity of the Pd center, and thus increases reactivity. The addition of an electron-donating substituent leads to a decrease in reactivity (PdL3 and PdL4). The reactivity of the nucleophiles depends on steric effects, with the bulky TMTU being the least reactive. The first and second substitution steps are associatively activated, given that the activation enthalpy are positive while the activation entropy are negative.
Data availability
The data is available in the form of supplementary information.
References
Muhammad N, Guo Z (2014) Metal-based anticancer chemotherapeutic agents. Curr Opin Chem Biol 19:144–153. https://doi.org/10.1016/j.cbpa.2014.02.003
Pinato O, Musetti C, Sissi C (2014) Pt-based drugs: the spotlight will be on proteins. Metallomics 6:380–395. https://doi.org/10.1039/c3mt00357d
Rosenberg B, Vancamp L, Trosko JE, Mansour VH (1969) Platinum compounds: a new class of potent antitumour agents. Nature 222:385–386. https://doi.org/10.1038/222385a0
Florea AM, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 3:1351–1371. https://doi.org/10.3390/cancers3011351
Qi L, Luo Q, Zhang Y et al (2019) Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol 32:1469–1486. https://doi.org/10.1021/acs.chemrestox.9b00204
Bugarčić ŽD, Bogojeski J, Petrović B et al (2012) Mechanistic studies on the reactions of platinum (II) complexes with nitrogen-and sulfur-donor biomolecules. Dalton Trans 41:12329–12345. https://doi.org/10.1039/C2DT31045G
Bugarčić ŽD, Bogojeski J, van Eldik R (2015) Kinetics, mechanism and equilibrium studies on the substitution reactions of Pd (II) in reference to Pt (II) complexes with bio-molecules. Coord Chem Rev 292:91–106. https://doi.org/10.1016/j.ccr.2015.02.016
Karami K, Mehvari F, Ramezanzade V et al (2022) The interaction studies of novel imine ligands and palladium (II) complexes with DNA and BSA for drug delivery application: the anti-cancer activity and molecular docking evaluation. J Mol Liq 362:119493. https://doi.org/10.1016/j.molliq.2022.119493
Montana AM, Batalla C (2009) The rational design of anticancer platinum complexes: the importance of the structure–activity relationship. Curr Med Chem 16:2235–2260. https://doi.org/10.2174/092986709788453087
Alam MN, Huq F (2016) Comprehensive review on tumour active palladium compounds and structure–activity relationships. Coord Chem Rev 316:36–67. https://doi.org/10.1016/J.CCR.2016.02.001
Alberto ME, Cosentino C, Russo N (2012) Hydrolysis mechanism of anticancer Pd (II) complexes with coumarin derivatives: a theoretical investigation. Struct Chem 23:831–839. https://doi.org/10.1007/s11224-011-9927-4
Divac VM, Mijatović A, Kostić MD, Bogojeski J (2017) The interaction of organoselenium trans-palladium (II) complexes toward small-biomolecules and CT-DNA. Inorganica Chim Acta 466:464–469. https://doi.org/10.1016/j.ica.2017.07.012
Jovanović S, Obrenčević K, Bugarčić ŽD et al (2016) New bimetallic palladium (II) and platinum (II) complexes: studies of the nucleophilic substitution reactions, interactions with CT-DNA, bovine serum albumin and cytotoxic activity. Dalton Trans 45:12444–12457. https://doi.org/10.1039/C6DT02226J
Ćoćić D, Jovanović S, Radisavljević S et al (2018) New monofunctional platinum (II) and palladium (II) complexes: studies of the nucleophilic substitution reactions, DNA/BSA interaction, and cytotoxic activity. J Inorg Biochem 189:91–102. https://doi.org/10.1039/C6DT02226J
Mitra I, Mukherjee S, Misini B et al (2018) Synthesis, biological evaluation, substitution behaviour and DFT study of Pd (II) complexes incorporating benzimidazole derivative. New J Chem 42:2574–2589. https://doi.org/10.1039/C7NJ05173E
Lazarević T, Rilak A, Bugarčić ŽD (2017) Platinum, palladium, gold and ruthenium complexes as anticancer agents: current clinical uses, cytotoxicity studies and future perspectives. Eur J Med Chem 142:8–31. https://doi.org/10.1016/j.ejmech.2017.04.007
Omondi RO, Fadaka AO, Fatokun AA et al (2022) Synthesis, substitution kinetics, DNA/BSA binding and cytotoxicity of tridentate N^ E^ N (E = NH, O, S) pyrazolyl palladium (II) complexes. J Biol Inorg Chem 27:653–664. https://doi.org/10.1007/s00775-022-01959-y
Omondi RO, Sibuyi NR, Fadaka AO et al (2021) Role of π-conjugation on the coordination behaviour, substitution kinetics, DNA/BSA interactions, and in vitro cytotoxicity of carboxamide palladium (ii) complexes. Dalton Trans 50:8127–8143. https://doi.org/10.1039/D1DT00412C
Omondi RO, Jaganyi D, Ojwach SO (2023) Electronic and ring size effects of N-heterocyclic carbenes on the kinetics of ligand substitution reactions and DNA/protein interactions of their palladium (II) complexes. BioMetals. https://doi.org/10.1007/s10534-023-00507-
Onunga DO, Bellam R, Mutua GK et al (2020) Controlling the reactivity of [Pd (II)(N^ N^ N) Cl]+ complexes using 2,6-bis (pyrazol-2-yl) pyridine ligands for biological application: substitution reactivity, CT–DNA interactions and in vitro cytotoxicity study. J Inorg Biochem 213:111261. https://doi.org/10.1016/j.jinorgbio.2020.111261
Kapdi AR, Fairlamb IJ (2014) Anti-cancer palladium complexes: a focus on PdX 2 L 2, palladacycles and related complexes. Chem Soc Rev 43:4751–4777. https://doi.org/10.1039/C4CS00063C
Zorbas H, Keppler BK (2005) Cisplatin damage: are DNA repair proteins saviors or traitors to the cell? ChemBioChem 6:1157–1166
Bogojeski J, Volbeda J, Freytag M et al (2015) Palladium (II) complexes with highly basic imidazolin-2-imines and their reactivity toward small bio-molecules. Dalton Trans 44:17346–17359. https://doi.org/10.1039/C5DT02307F
Bravin C, Badetti E, Licini G, Zonta C (2021) Tris (2-pyridylmethyl) amines as emerging scaffold in supramolecular chemistry. Coord Chem Rev 427:213558. https://doi.org/10.1016/j.ccr.2020.213558
Tripathi RP, Saxena N, Tiwari VK et al (2006) Synthesis and antitubercular activity of substituted phenylmethyl-and pyridylmethyl amines. Bioorg Med Chem 14:8186–8196. https://doi.org/10.1016/j.bmc.2006.09.020
Gómez J, García-Herbosa G, Cuevas JV et al (2006) Diastereospecific and diastereoselective syntheses of ruthenium (II) complexes using N,N′ bidentate ligands aryl-pyridin-2-ylmethyl-amine ArNH-CH2-2-C5H4N and their oxidation to imine ligands. Inorg Chem 45:2483–2493. https://doi.org/10.1021/ic051590a
Kim S, Kim D, Lee H-J, Lee H (2014) Palladium (II) complexes containing N,N′-bidentate N-(pyridin-2-ylmethyl) aniline and its derivatives: synthesis, structural characterisation, and methyl methacrylate polymerisation. Polyhedron 77:66–74. https://doi.org/10.1016/j.poly.2014.04.015
Schiessl WC, Summa NK, Weber CF et al (2005) Experimental and theoretical approaches to the protonation of thiourea: a convenient nucleophile in coordination chemistry revisited. Z Anorg Allg Chem 631:2812–2819. https://doi.org/10.1002/zaac.200500157
Frisc MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09 (Revision C.01). Gaussian, Wallingford. www.gaussian.com
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310. https://doi.org/10.1063/1.448975
Becke AD (1986) Density functional calculations of molecular bond energies. J Chem Phys 84:4524–4529. https://doi.org/10.1063/1.450025
Mundinger S, Jakob U, Bichovski P, Bannwarth W (2012) Modification and optimization of the bis-picolylamide-based relay protection for carboxylic acids to be cleaved by unusual complexation with Cu2+ salts. J Org Chem 77:8968–8979. https://doi.org/10.1021/jo301349t
Park S, Lee JK, Lee H (2019) Zinc (II), palladium (II) and cadmium (II) complexes containing 4-methoxy-N-(pyridin-2-ylmethylene) aniline derivatives: synthesis, characterization and methyl methacrylate polymerization. Appl Organomet Chem 33:e4797. https://doi.org/10.1002/aoc.4797
Lin YC, Yu KH, Huang SL et al (2009) Alternating ethylene-norbornene copolymerization catalyzed by cationic organopalladium complexes bearing hemilabile bidentate ligands of α-amino-pyridines. Dalton Trans 41:9058–9067. https://doi.org/10.1039/B912068H
Diez V, Cuevas JV, García-Herbosa G et al (2007) 1H NMR direct observation of enantiomeric exchange in palladium (II) and platinum (II) complexes containing N,N′ bidentate aryl-pyridin-2-ylmethyl-amine ligands. Inorg Chem 46:568–577. https://doi.org/10.1021/ic061060u
Al-Allaf T, Castan P, Turpin R et al (1992) Solvolysis of palladium (II) and platinum (II) complexes of asymmetric ligands: synthesis and structural characterization of [Pd(AMP)(dmso)Cl]BF4 and [Pt (AMP)(dmso) Cl] ClO4. Transit Met Chem 17:579–582. https://doi.org/10.1007/BF02910763
Ghosh GK, Misra K, Linert W, Moi SC (2013) Interaction of glutathione with cis-(2-aminomethylpyridine) diaqua platinum (II) perchlorate in aqueous medium: their kinetics and mechanism. Synth React Inorg Met Nano-Met Chem 43:714–721. https://doi.org/10.1080/15533174.2012.754760
Summa N, Schiessl W, Puchta R (2006) Thermodynamic and kinetic studies on reactions of Pt (II) complexes with biologically relevant nucleophiles. Inorg Chem 45:2948–2959. https://doi.org/10.1021/ic051955r
Hochreuther S, Puchta R, van Eldik R (2011) Novel dinuclear platinum (II) complexes containing mixed nitrogen–sulfur donor ligands. Inorg Chem 50:12747–12761. https://doi.org/10.1021/ic2018902
Bera SK, Chandra SK, De SG (2003) Kinetics of substitution of aqua ligands from cis-diaqua (ethylene-diamine)platinum(II) perchlorate by l-asparagine in aqueous medium. Int J Chem Kinet 35:252–259. https://doi.org/10.1039/C1DT11453K
Ghosh S, Sengupta P, De GS (1999) Kinetics and mechanism of the interaction of dl-methionine with cis-diaquaethylenediamine platinum (II) perchlorate in aqueous medium. Transit Met Chem 24:59–63. https://doi.org/10.1023/A:1006993423954
Hochreuther S, Nandibewoor ST, Puchta R, van Eldik R (2012) Thermodynamic and kinetic behaviour of [Pt (2-methylthiomethylpyridine)(OH2)2]2+. Dalton Trans 41:512–522. https://doi.org/10.1039/C1DT11453K
Papo TR, Jaganyi D, Mambanda A (2022) Substitution reactions of cis-platinum (II) complexes containing bidentate N,N-donor pyridinecarboxamide ligands with different substituents. J Coord Chem 75:2557–2573. https://doi.org/10.1080/00958972.2022.2149327
Kinunda G, Jaganyi D (2014) A kinetic study of aqua ligand substitution in dinuclear Pt (II) complexes containing four non-coplanar pyridine ligands. Transit Met Chem 39:939–949. https://doi.org/10.1007/s11243-014-9879-9
Hochreuther S, Puchta R, van Eldik R (2011) Thermodynamic and kinetic studies on novel dinuclear platinum (II) complexes containing bidentate N,N-donor ligands. Inorg Chem 50:8984–8996. https://doi.org/10.1021/ic201151h
Hohmann H, Hellquist B, Van Eldik R (1991) Effect of steric hindrance on kinetic and equilibrium data for substitution reactions of diaqua (N-substituted ethylenediamine) palladium (II) with chloride in aqueous solution. Inorg Chim Acta 188:25–32. https://doi.org/10.1016/S0020-1693(00)80912-3
Acknowledgements
The authors gratefully acknowledge financial support from the University of KwaZulu-Natal and the National Research Foundation (NRF, South Africa).
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mjwara, P.N., Papo, T.R. & Sithebe, S. The influence of electronic effects on the substitution reactions of N,N′-donor cis-palladium(II) complexes with bidentate (pyridin-2-yl)methyl-aniline ligands. Reac Kinet Mech Cat 136, 2907–2928 (2023). https://doi.org/10.1007/s11144-023-02504-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02504-x