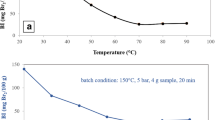
Abstract
In this investigation, a low-cost catalyst was prepared using a natural Maghnia sodic bentonite submitted to a simple ion exchange process and calcination at 500 °C for 3 h. This material was used in the removal of methyl orange (MO) dye by heterogeneous Fenton oxidation. The chemical composition was quantified by XRF and EDX and showed a significant increase in the iron content indicating a successful insertion of the iron within the clay reaching 5.98 wt%. Furthermore, XRD and FTIR analysis showed that the clay preserved its crystalline structure with the presence of montmorillonite as the main phase with some loss of characteristic peaks related to hydroxyl groups due to the calcination. Moreover, the specific surface area increased from 56 to 86 m2/g after the ion exchange treatment. High catalytic activity was reached, achieving the complete decolorization of the dye solution after only 10 min at T = 65 °C, [H2O2] = 9.8 mM and mcatalyst = 1 g/L. The kinetic constant rates were calculated by adjusting the experimental data to the nonlinear first-order model. The positive value of enthalpy variation (∆H° = 23.70 kJ/mol) indicated the endothermic nature of the process. The reaction rate was also found to be controlled by diffusion with an activation energy smaller than 29 kJ/mol (Ea = 26.35 kJ/mol). The reusability of the catalyst was also examined showing high degradation efficiency (90%) even after 8 cycles. The catalyst showed low iron leaching during reaction experiments even in an acidic medium at 65 °C.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon request.
References
Kıpçak İ, Ersal EK (2021) Catalytic wet peroxide oxidation of a real textile azo dye Cibacron Red P-4B over Al/Fe pillared bentonite catalysts: kinetic and thermodynamic studies. React Kinet Mech Catal 132:1003–1023. https://doi.org/10.1007/s11144-021-01962-5
Xu HY, Wang Y, Shi TN, Zhao H, Tan Q, Zhao BC, He XL, Qi SY (2018) Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNTs as catalyst: kinetics and Fenton-like mechanism. Front Mater Sci 10:45–55. https://doi.org/10.1007/s11706-018-0412-5
Galeano LA, Gil A, Vicente MA (2010) Effect of the atomic active metal ratio in Al/Fe-, Al/Cu and Al/(Fe–Cu)-intercalating solutions on the physicochemical properties and catalytic activity of pillared clays in the CWPO of methyl orange. Appl Catal B: Environ 100:271–281. https://doi.org/10.1016/j.apcatb.2010.08.003
Filice S, Bongiorno C, Libertino S, Gradon L, Iannazzo D, Scalese S (2022) Photo-Fenton degradation of methyl orange with Dunino Halloysite as a source of iron. Catalysts 12(3):1–16. https://doi.org/10.3390/catal12030257
Koul B, Yadav D, Singh S, Kumar M, Song M (2022) Insights into the domestic wastewater treatment (DWWT) regimes: a review. Water 14(21):1–29. https://doi.org/10.3390/w14213542
Shokri A, Salimi M, Abmatin T (2017) Employing photo Fenton and UV/ZnO processes for removing reactive red 195 from aqueous environment. Fresenius Environ Bull 26(2):1560–1565. https://doi.org/10.1016/j.surfin.2020.100705
Shokri A, Mahanpoor K (2016) Removal of ortho-toluidine from industrial wastewater by UV/TiO2 process. J Chem Health Risks 6(3):213–223. https://doi.org/10.1016/j.envc.2021.10033
Shokri A, Karimi S (2020) Treatment of aqueous solution containing acid red 14 using an electro peroxone process and a Box-Behnken experimental design. Arch. Hyg. Sci 9(1):48–57
Shokri A, Sanavi FM (2022) Using α-Fe2O3/SiO2 as a heterogeneous Fenton catalyst for the removal of chlorophenol in aqueous environment: thermodynamic and kinetic study. IJEST 20:383–396. https://doi.org/10.1007/s13762-022-04498-w
Arshadi M, Abdolmaleki MK, Mousavinia F, Khalafi-Nezhad A, Firouzabadi H, Gil A (2016) Degradation of methyl orange by heterogeneous Fenton-like oxidation on a nano-organometallic compound in the presence of multi-walled carbon nanotubes. Chem Eng Res Des 112:113–121. https://doi.org/10.1016/j.cherd.2016.05.028
Li C, Zhang TC, Liang J, Liang Y (2019) Removal of methyl orange wastewater by heterogeneous Fenton-like reaction over activated carbon pre-treated by nitric acid. Desalin Water Treat 145:393–398. https://doi.org/10.5004/dwt.2019.23738
Jian L, Peng G, Jing X, Yi Z (2018) Treatment of methyl orange by the catalytic wet peroxide oxidation process in batch and continuous fixed bed reactors using Fe-impregnated 13X as catalyst. Water Sci Technol 78(4):936–946. https://doi.org/10.2166/wst.2018.372
Arroyo-Gómez JJ, Toncón-Leal CF, dos Santos AJ, Moreno MS, Sapag K, Martínez-Huitle CA (2020) Fe/SBA-15: characterization and its application to a heterogeneous solar photo-Fenton process to decolorize and mineralize an azo dye. Mater Lett 5:100034. https://doi.org/10.1016/j.mlblux.2019.100034
Boukhemkhem A, Rida K, Pizarro AH, Molina CB, Rodriguez JJ (2019) Iron catalyst supported on modified kaolin for catalytic wet peroxide oxidation. Clay Miner 54(1):67–73. https://doi.org/10.1180/clm.2019.9
Li Y, Li Y, Lv J, Zhao Z, Sun G (2022) Heterogeneous Fenton degradation of methyl orange using Fe–Al–Ce bentonite as catalyst. Russ J Phys Chem 96:302–308. https://doi.org/10.1134/S0036024422020303
Huang GQ, Qi GJ, Gao TY, Zhang J, Zhao YH (2020) Fe-pillared montmorillonite as effective heterogeneous Fenton catalyst for the decolorization of methyl orange. J Chem Sci 116(132):1–8. https://doi.org/10.1007/s12039-020-01820-2
Gil A, Korili SA, Trujillano R, Vicente MA (2010) Pillared Clays and Related Catalysts. Springer, New York
Tomul F (2012) Adsorption and catalytic properties of Fe/Cr-pillared bentonites. J Chem Eng 185(1865):380–390. https://doi.org/10.1016/j.cej.2012.01.094
Chae HJ, Nam IS, Ham SW, Hong SB (2001) Physicochemical characteristics of pillared interlayered clays. Catal Today 68:31–40. https://doi.org/10.1016/S0920-5861(01)00320-0
Palinkou I, Lazar K, Kiricsi I (1997) Cationic mixed pillared layer clays: infrared and Mössbauer characteristics of the pillaring agents and pillared structures in Fe, Al and Cr, Al pillared montmorillonites. J Mol Struct 410–411:547–550. https://doi.org/10.1016/S0022-2860(96)09516-6
Mudrinić TM, Ajduković MJ, Jović-Jovicić NP, Marinović SR, Mojović ZD, Milutinović-Nicolić AD, Banković PT (2018) Al, Fe, Ni-pillared bentonite in the catalytic wet peroxide oxidation of the textile dye acid yellow 99. React Kinet Mech Catal 124:75–88. https://doi.org/10.1007/s11144-018-1386-0
Henao-Aguire PA, Macías-Quiroga IF, Giraldo-Gómez GI, Sanabria-González NR (2021) Catalytic oxidation of Ponceau 4R in aqueous solution using iron-impregnated Al-pillared bentonite: optimization of the process. Bull Chem React Eng 16(3):491–506. https://doi.org/10.9767/bcrec.16.3.10757.491-506
Thuan NT, Khoi TT, Chi NM, Vinh NN (2020) Removal of methyl orange by heterogeneous Fenton process using iron dispersed on alumina pillared bentonite pellet. J Sci Technol 23(2):555–563. https://doi.org/10.32508/stdj.v23i2.2139
Saywell LG, Cunningham BB (1937) Determination of iron: colorimetric o-phenanthroline method. Ind Eng Chem Res 9(2):67–69. https://doi.org/10.1021/ac50106a005
Boukhemkhem A, Pizarro AH, Molina CB (2020) Enhancement of the adsorption properties of two natural bentonites by ion-exchange: equilibrium, kinetics and thermodynamic study. Clay Miner 55:132–141. https://doi.org/10.1180/clm.2020.19
Tomić ZP, Logar VP, Babić BM, Rogan JR, Makreski P (2011) Comparison of structural, textural and thermal characteristics of pure and acid treated bentonites from Aleksinac and Petrovac (Serbia). Spectrochim Acta A Mol 82:389–395. https://doi.org/10.1016/j.saa.2011.07.068
Guo S, Zhang G, Wang J (2014) Photo-Fenton degradation of rhodamine B using Fe2O3-Kaolin as heterogeneous catalyst: characterization, process optimization and mechanism. J Colloid Interface Sci 433:1–8. https://doi.org/10.1016/j.jcis.2014.07.017
Khanikar N, Bhattacharyya KG (2013) Cu(II)-kaolinite and Cu(II) montmorillonite as catalyst for wet oxidative degradation of 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. J Chem Eng 233:88–97. https://doi.org/10.1016/j.ultsonch.2016.08.017
Chen J, Zhu L (2009) Comparative study of catalytic activity of different Fe-pillared bentonites in the presence of UV light and H2O2. Sep Purif Technol 67:282–288. https://doi.org/10.1016/j.seppur.2009.03.036
Christidis GE, Scott PW, Dunham AC (1997) Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean, Greece. Appl Clay Sci 12:329–347. https://doi.org/10.1016/S0169-1317(97)00017-3
Daud NK, Ahmad MA, Hameed BH (2011) Decolorization of acid red 1 dye solution by Fenton-like process using Fe–Montmorillonite K10 catalyst. J Chem Eng 165:111–116. https://doi.org/10.1016/j.cej.2010.08.072
Groeningen NV, Thomas Arrigo LK, Byrne JM, Kappler A, Cristl I, Kretzschmar R (2020) Interactions of ferrous iron with clay mineral surfaces during sorption and subsequent oxidation. Environ Sci Process Impacts 22:1355–1367. https://doi.org/10.1039/D0EM00063A
Duan F, Yang Y, Li Y, Cao H, Wang Y, Zhang Y (2014) Heterogeneous Fenton-like degradation of 4-chlorophenol using iron/ordered mesoporous carbon catalysts. Environ Sci 26:1171–1179. https://doi.org/10.1016/S1001-0742(13)60532-X
Carriazo J, Guelou E, Barrault J, Tatibouet JM, Molina R, Moreno S (2005) Catalytic wet peroxide oxidation of phenol by pillared clays containing Al–Ce–Fe. Water Res 39:3891–3899. https://doi.org/10.1016/j.watres.2005.06.034
Han J, Zeng H-Y, Xu S, Chen C-R, Liu X-J (2016) Catalytic properties of CuMgAlO catalyst and degradation mechanism in CWPO of methyl orange. Appl Catal A-Gen 527:72–80. https://doi.org/10.1016/j.apcata.2016.08.015
Hassan H, Hameed BH (2011) Fe-clay as effective heterogeneous Fenton catalyst for the decolorization of reactive blue 4. J Chem Eng 171:912–918. https://doi.org/10.1016/j.cej.2011.04.040
Lente G, Fábián I, Poë AJ (2005) A common misconception about the Eyring equation. New J Chem 29:259–260. https://doi.org/10.1039/B501687H
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boukhemkhem, A., Hameurlaine, S., Molina, C.B. et al. Efficient degradation of methyl orange by heterogeneous Fenton reaction using modified bentonite by a simple method. Reac Kinet Mech Cat 136, 3173–3190 (2023). https://doi.org/10.1007/s11144-023-02498-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02498-6