Abstract
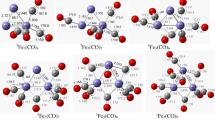
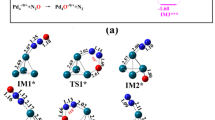
Density functional theory is employed to investigate the conversion of N2O and CO on Fe6M (M = Fe, Co, Ni, Mn) clusters. Two reaction mechanisms are established in this work: the stepwise adsorption mechanism and the co-adsorption adsorption mechanism. The N- and O-bound adsorption of N2O are both considered in two mechanisms. The average binding energy declares the doping of Mn and Ni atoms enhanced the stability of clusters. The potential energy curves verify that CO oxidation to form CO2 is the rate-determining step for two mechanisms, and the co-adsorption mechanism is both thermodynamically and kinetically favorable compared with the stepwise adsorption mechanism. Among the investigated clusters, the Fe6Co(t) cluster exhibits the superior catalytic activity for the conversion of N2O and CO reaction with the lowest energy barrier (23.45 kcal/mol) for the rate-determining step.




Similar content being viewed by others
References
Hoekman SK (2020) Review of nitrous oxide (N2O) emissions from motor vehicles. SAE Int J Fuels Lubr 13:79–98
Ravishankara AR, Da Niel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Kappes MM, Staley RH (1981) Gas-phase oxidation catalysis by transition-metal cations. J Am Chem Soc 103:1286–1287
Nematollahi P, Esrafili MD (2016) A DFT study on the N2O reduction by CO molecule over silicon carbide nanotubes and nanosheets. RSC Adv 6:59091–59099
Yutthana W, Supawadee N, Nawee K, Siriporn J (2017) Mechanistic study of CO oxidation by N2O over Ag7Au6 cluster investigated by DFT methods. Appl Catal A 538:99–106
Yang H, Fu H, Su B, Xiang B, Hu CW (2015) Theoretical study on the catalytic reduction mechanism of NO by CO on tetrahedral Rh4 subnanocluster. J Phys Chem A 119:11548–11564
Lian X, Guo WL, He B, Yu B, Chen SQ, Qin D, Chen FL (2020) Insights of the mechanisms for CO oxidation by N2O over M@Cu12 (M = Cu, Pt, Ru, Pd, Rh) core–shell. Mol Catal 494:111126
Zhdanov VP, Nakagoe O, Matsushima T (2007) Kinetics of the N2O-CO reaction on Rh (110). Surf Sci 601:L49–L54
Granger P, Malfoy P, Esteves P, Leclercq L, Leclercq G (1999) Kinetics of the CO+N2O reaction over noble metals: I. Pt/Al2O3. J Catal 187:321–331
Chen X, Yimeng L, Uzoma N, Schwank JW (2018) Reactivity study of CO+NO reaction over Pd/Al2O3 and Pd/CeZrO2 catalysts. Catal Today 323:148–158
Delahay G, Mauvezin M, Guzman-Vargas A, Coq B (2002) Effect of the reductant nature on the catalytic removal of N2O on Fe-zeolite-catalysts. Catal Commun 3:385–389
Pérez-Ramı́rez J, Kumar MS, Brückner A (2004) Reduction of N2O with CO over FeMFI zeolites: influence of the preparation method on the iron species and catalytic behavior. J Catal 223:13–27
Boutarbouch MN, Cortes JM, Begrani MS, Lecea CS, Pérez-Ramı́rez J (2004) Catalytic conversion of N2O over FeZSM-5 zeolite in the presence of CO and NO. Appl Catal B 54:115–123
Wu SY (2011) Adsorption and dissociation of N2O molecule on Fe(111) surface: a DFT study. Comput Mater Sci 50:3311–3314
Dai C, Lei Z, Wang Y (2013) Reduction of N2O by CO over Fe- and Cu-BEA zeolites: an experimental and computational study of the mechanism. Microporous Mesoporous Mater 167:254–266
Debbagh MN, Bueno-López A, Lecea CSMD, Pérez-Ramírez J (2007) Kinetics of the N2O+CO reaction over steam-activated FeZSM-5. Appl Catal A 327:66–72
Popolan DM, Bernhardt TM (2011) Communications: CO oxidation by silver and gold cluster cations: identification of different active oxygen species. J Chem Phys 134:91–102
Hirabayashi S, Ichihashi M (2014) Catalytic oxidation of CO with N2O on isolated copper cluster anions. Phys Chem Chem Phys 16:26500–26505
Balaj OP, Balteanu I, RoßTeuscher T (2004) Catalytic oxidation of CO with N2O on gas-phase platinum clusters. Angew Chem Int Ed 43:6519–6522
Yamada A, Miyajima K, Mafuné F (2012) Catalytic reactions on neutral Rh oxide clusters more efficient than on neutral Rh clusters. Phys Chem Chem Phys 14:4188–4195
Su BF, Hu CW, Fu HQ, Yang HQ (2015) Catalytic reduction of NO by CO on Rh4+ clusters: a density functional theory study. Catal Sci Technol 5:3203–3215
Barabás J, Höltzl T (2016) Reaction of N2O and CO catalyzed with small copper clusters: mechanism and design. J Phys Chem A 120:8862–8870
Fang HL, Xu L, Li J, Wang B, Zhang YF, Huang X (2015) Catalytic oxidation of CO by N2O on neutral Y2MO5 (M = Y, Al) clusters: a density functional theory study. RSC Adv 5:76651–76659
Wang ZC, Yin S, Bernstein ER (2013) Catalytic oxidation of CO by N2O conducted via the neutral oxide cluster couple VO2/VO3. Phys Chem Chem Phys 15:10429–10434
Mi H, Wei SH, Duan XM, Pan XY (2015) Catalytic reduction of N2O by CO over PtlAum clusters: a first-principles study. Chin Phys B 24:557–561
Han YX, Kong C, Hou LJ, Chen DP, Geng ZY (2017) Theoretical research on the catalytic reaction mechanism of N2O and CO over Ni5 cluster. Comput Theor Chem 1117:12–19
Kim E, Mohrland A, Weck PF, Tao P, Tománek D (2014) Magic numbers in small iron clusters: a first-principles study. Chem Phys Lett 613:59–63
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. Revision B.01.01. Gaussian Inc., Wallingford
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys Rev A 38:3098–3100
Lee CT, Yang WT, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron-density. Phys Rev B 37:785–789
Becke AD (1998) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Hay PJ, Wadt WR (1984) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:284–299
Raghavachari K, Binkley JS, Seeger R, People JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Kartha KK, Pai MR, Banerjee AM, Pai RV, Meena SS, Bharadwaj SR (2011) Modified surface and bulk properties of Fe-substituted lanthanum titanates enhances catalytic activity for CO + N2O reaction. J Mol Catal A 335:158–168
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 21903009), and Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN202101517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, H., Zeng, W., Tang, X. et al. Theoretical investigation for the reaction of CO oxidation by N2O on Fe6M (M = Fe, Co, Ni, Mn) clusters. Reac Kinet Mech Cat 135, 3021–3030 (2022). https://doi.org/10.1007/s11144-022-02308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02308-5