Abstract
We explored the isomerization of d-glucose into d-fructose using the simplest possible base catalyst, aqueous NaOH, to maintain a constant pH value during the reaction. Under the applied mild conditions (T 50–90 °C, pH 9.5–11.5), yields of d-fructose of up to 31% were observed. Selectivity-conversion plots were not significantly influenced by variation of the temperature, pH value or substrate concentration. A reaction network for kinetic modelling includes d-glucose-d-fructose interconversion, co-production of d-mannose and d-allulose (also known as d-psicose) as well as decomposition paths after deprotonation of the hexoses. All four hexoses were employed as substrates in the isomerization. Thermodynamic ionization constants of the saccharides were measured by means of potentiometric titration. In the kinetic studies, pH-independent rate constants as well as activation energies were determined. The obtained kinetic and thermodynamic results as well as selectivity-conversion correlations present a useful benchmark for soluble and solid base catalysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemo catalysts for the isomerization
Isomerization of d-glucose (Glc) into d-fructose (Fru) is implemented on the industrial level for production of high-fructose corn syrups for food industry [1, 2]. This process presents one of the most optimized and the largest biotechnological manufactures. Additionally, Fru has been indicated as an important intermediate for valorization of lignocellulosic biomass [3,4,5]. In this regard, recent studies have been focused on development of chemo catalysts, which potentially exhibit lower costs and higher stability compared to enzymes [3, 5,6,7].
Catalytic activity of bases for the Glc-Fru isomerization has been known since the nineteenth century [6]. In early works, catalytic performance of soluble bases was studied and the reaction mechanism, which is shown in Scheme 1, was proposed. In 2000, Moreau et al. published the first paper aiming at developing a solid base catalyst for the Glc–Fru isomerization [8]. Since then, catalytic activity of a large number of basic solids was reported including MgO [9,10,11,12,13] and other alkaline earth (hydr)oxides [14], Mg–Al hydrotalcites [9, 10, 15,16,17,18,19,20,21,22,23,24], Mg- or Ca-impregnated or exchanged zeolites [25,26,27,28,29], attapulgite [30, 31], MgO–Nb phosphate [32], Mg-doped carbon nitride [33], Mg-containing titanosilicates [34], CaO–ZrO2 [35], MgO–ZrO2 [36], CaO–Al2O3 [37], CaO–MgO [38], CaO/C [39], alkaline earth metal titanates [40], soluble amines [41,42,43,44,45,46,47,48,49,50,51] and N-containing solid catalysts [43, 44, 47, 48, 52,53,54,55,56], silicates [57], SiO2 treated with ammonia [58], zirconium carbonate [59], ZrO2 [60], basic hybrid catalysts [61], and basic salts with low solubility [62]. We have recently shown that catalytic activity of basic low-soluble (hydr)oxides [14] and basic salts [62] in aqueous medium relies on partial dissolution of the materials releasing OH− species. The latter acts as in situ generated catalytically active species. Owing to dissolution and other reasons—such as adsorption of by-products on the catalyst surface—solid base catalysts deactivate on stream, as suggested by the results of studies in continuous reactors [8, 9, 15, 63].
Solid Lewis acids, especially Sn-Beta zeolites, exhibit high catalytic activity for the isomerization [64]. Unfortunately, these materials also show quick deactivation on stream owing to leaching of Sn and structural changes of the zeolites [65]. To our knowledge, only Hf-containing zeolites exhibit high stability for the Glc–Fru isomerization under continuous conditions [66]. Unfortunately, the reported data on stability were obtained using very low Glc concentration of 1 wt% and methanol as solvent, whereas higher substrate concentrations and aqueous media are of greater interest from practical viewpoint.
Kinetics and mechanism
Kinetics and mechanism of the isomerization were intensively studied and discussed in the twentieth century. Finally, a generally accepted mechanism was formulated [67, 68]. Scheme 1 shows the major steps of the mechanism, which includes (1) Glc deprotonation in the presence of base, (2) ring opening of Glc anion as a quasi-equilibrium step with a dominance of a cyclic form (a share of an open form was estimated as 5% [67, 69]), and (3) formation of the 1,2-enediol intermediate as the rate-determining step. Subsequently, (4) Fru is formed from the 1,2-enediol (ED1,2), undergoes (5) ring closing and (6) re-protonation. This mechanism was supported by operando UV investigations [67, 70], incorporation of deuterium and tritium using D2O or T2O as solvents [70, 71], 13C NMR studies [69], kinetic isotope effect [42, 71], and structure–reactivity correlations [70].
ED1,2 presents a highly reactive species, which is present in a very low concentration that can hardly be measured [67, 68]. Therefore, ED1,2, \({\mathrm{Glc}}_{\mathrm{open}}^{-}\), and \({\mathrm{Fru}}_{\mathrm{open}}^{-}\) are assumed to be in quasi-equilibrium with the other species and Scheme 1 is simplified according to Scheme 2 [68].
Glc− and Fru− correspond here to the lump concentrations of saccharide anions in open and closed form; KGlc and KFru designate thermodynamic ionization constants of the hexoses, and kA and k-A are efficient isomerization constants. Moreover, deprotonation of hexoses is assumed to proceed significantly more rapidly than the isomerization and the closed hexose-anions are also treaded as being present in a quasi-equilibrium [68].
In accordance with Scheme 2, Eqs. 1 and 2 give the expressions for the rates of Glc consumption and Fru formation, respectively:
Analysis of the reaction mixture by chromatographic methods such as GC or HPLC provides information about a combined concentration (\({c}_{\mathrm{Hex total}}\)) for the hexoses, consisting of a hexose in its protonated (\(\left[\mathrm{Hex}\right]\)) and deprotonated (\(\left[{\mathrm{Hex}}^{-}\right]\)) form (Eq. 3) [72].
A ratio between protonated and deprotonated forms of a hexose can be calculated using an ionization constant of a hexose \({K}_{\mathrm{Hex}}\) according to Eq. 4 [68, 72].
Consequently, the total hexose concentration can be expressed according to Eq. 5.
The latter can be rearranged to yield an expression for \(\left[{\mathrm{Hex}}^{-}\right]\) (Eq. 6) [72].
Substituting these expressions for the hexose anions into the Eqs. 1 and 2 results in Eqs. 7 and 8 (Scheme 3).
According to Eqs. 7 and 8, apparent rate constants contain pH-independent (kA and k-A) and pH-dependent \(\left(\frac{{K}_{\mathrm{Glc}}\left[{\mathrm{OH}}^{-}\right]}{1+{K}_{\mathrm{Glc}}\left[{\mathrm{OH}}^{-}\right]} \mathrm{and }\frac{{K}_{\mathrm{Fru}}\left[{\mathrm{OH}}^{-}\right]}{1+{K}_{\mathrm{Fru}}\left[{\mathrm{OH}}^{-}\right]}\right)\) terms. For the first time, Kooyman et al. published pH-independent rate constants for the isomerization catalyzed by NaOH. Moreover, the authors consolidated all the previously published data and calculated the corresponding pH-independent rate constants [68]. More recent publications on kinetics of isomerization in the presence of triethylamine [42] or solid catalysts [35, 38] do not provide data on pH-independent rate constants.
Though the majority of the kinetic studies exploit Scheme 2 as a reaction network, it is oversimplified omitting side products, such as d-mannose (Man) and d-Allulose (Allu, also referred to as d-Psicose) as well as decomposition products. de Bruijn et al. performed kinetic modeling using a reaction network, which included four saccharides Glc, Fru, Man, and Allu, using more diluted KOH solution in the concentration range of 10–3 to 10–1 M. However, the authors did not report temperature dependence of the reaction constants and determined apparent rate constants, consisting of both deprotonation and isomerization [73].
In this work, we explored Glc–Fru isomerization using NaOH as catalyst, which was added continuously for keeping a constant pH value. This simple system was applied to obtain kinetic parameters of the isomerization, such as pH-independent reaction rate constants and activation energies. According to our knowledge, these kinetic data are not available in literature though they are very useful as a benchmark for solid base catalysts.
In addition, our results suggest that NaOH is comparable to most of solid catalysts or more complex soluble catalysts in terms of selectivity for Fru and product yield. Thus, NaOH can be considered as a chemo catalyst for the Glc–Fru isomerization—coupled for example with electrodialysis—unless robust solid catalysts are uncovered.
Experimental and methods
Chemicals
Chemicals were used without further purification. d-fructose (≥ 99.5%), sodium bicarbonate (≥ 99%), and acetone (≥ 99.5%) were purchased from Roth. Amberlyst 15 (H+ Form) was acquired from Sigma-Aldrich. Hydrochloric acid (0.1 N), desiccant (Silica gel, granules, Cobalt free), and sodium hydroxide (≥ 99.5%) were sourced from Chemsolute. n-Tetradecan (≥ 99%) and Amberlite IRA-96 (free base) were obtained from Alfa Aesar. Sulfuric acid (98%) and D-glucose (≥ 99%) were purchased from Merck. d-Mannose (99%) was obtained from abcr. d-allulose (> 98%) was provided by Pfeifer & Langen GmbH. Buffer solutions at pH 4.01, 7.00, 10.01 were sourced from Hamilton. Fill type solution L300 and storage solution L911 for the glass electrode were obtained from SI Analytics. All solutions were prepared with desalinated water.
Titrator and electrodes
All titrations were conducted on a TitroLine 7000 titrator from SI Analytics, equipped with a WA 20 exchange unit or on a TitroLine alpha plus titrator from SI Analytics equipped with a TW 20 exchange unit. The titrators were connected to an IoLine-pHT-A120MF-DIN-N electrode, which was filled with L300 fill type solution, or SteamLine 81-120 pHT VP electrodes. The electrodes were stored in L911 storage solution when not in use. Before each use, the electrodes were calibrated using buffer solutions (pH 4.01, 7.00, 10.01).
Kinetic measurements
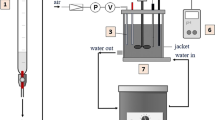
Kinetic measurements were conducted in a 250 mL 4-neck round bottom flask. The 4-neck flask was equipped with a T-shape stirring rod, powered by a Heidolph RZR 2041 overhead stirrer, a reflux condenser, a titration tip, and an electrode. The hexose solutions were prepared by dissolving the hexoses in an appropriate amount of water to yield 200 g of reactant solution. From this solution, 150 mL were transferred into the reaction flask. The reaction mixture was heated to the corresponding reaction temperature in an oil bath, upon stirring at 400 rpm. Afterwards, the pH value was set using the auto titrator equipped either with 1 M NaOH to keep pH 9.5 or 10.5 or 3 M NaOH to adjust pH to 11.5. The autotitrator was employed to keep the pH value constant during the experiments. Once the pH value was set, a sample of 2 mL was taken from the reaction mixture and cooled in an ice bath to stop the reaction. This sample was designated as the initial one at treaction = 0 min. Afterwards, samples were collected in regular intervals. The temperature in the reaction flask was measured after completion of the experiment by the temperature sensor in the pH electrode. Analysis was carried out by gas chromatography after desalination and subsequent derivatization.
Analysis by gas chromatography
1 mL of a cooled sample was diluted with 9 mL of water. Consequently, the resulting solution was treated two times each alternating between Amberlyst 15 H+ form (200 mg, 30 min) and Amberlite IRA-96 free base (1000 mg, 60 min) ion exchange resins to remove side products and to neutralize the solution. Afterwards, the samples were passed through a Chromafil Xtra PA-20/25 syringe filter from Macherey–Nagel. 1 mL of the diluted and desalinated samples was frozen using liquid N2 and dried in vacuo over silica gel desiccant. To facilitate analysis by means of gas chromatography, the hexoses were acetonated using a modified procedure proposed by Ekeberg et al. [74]. First, approximately 14 mg of n-tetradecane were added to the dried sample as an internal standard and the exact mass was recorded. Then, the sample was treated with 2.5 mL of 2 mol% H2SO4 in acetone for 2.5 h. Neutralization with NaHCO3 (approx. 600 mg) and subsequent filtration through a Chromafil Xtra PE-45/25 syringe filter from Macherey–Nagel resulted in a colorless sample used for analysis.
Analysis of the product mixture was carried out on an HP 6890 gas chromatograph, employing a Machery-Nagel Optima 17-MS column (30 m × 0.25 µm) and an FID detector. Starting at 80 °C, the temperature was increased to 250 °C at 12 °C·min−1. The hexose concentration was calculated using calibration factors and the signal of the internal standard n-tetradecane at 6.3 min as reference. The following hexoses were analyzed: Allu (9.3 min), Glc (10.6 min), Man (11.1 min), and Fru (10.1 min and 10.7 min). The areas of the signals corresponding to Fru were combined for calibration as well as calculation of the concentration.
pKa measurements
Measurements were conducted in 5 °C intervals from 10 to 30 °C in a 100 mL 3-neck round bottom flask, equipped with a turbine shape stirring rod, powered by a Heidolph RZR 2041 overhead stirrer, a cannula, a titration tip, and an electrode. Heating/cooling was performed by a water bath inside a glass jacketed vessel of 600 mL volume, which was connected to a Corio 200F thermostat (Julabo). Before the titration, the 0.1 M hexose solutions were prepared by dissolving the hexose in 50 mL of water and removing residual CO2 by passing Ar through the solution for 15 min. Once the desired temperature was reached inside the flask, 0.5 M aq. NaOH was added in increments of 0.2 mL until an added volume of 14 mL was reached. The pH value was measured after each step. During the titration, the solution was stirred at 700 rpm and a stream of argon was passed through the solution via the cannula. The exact concentration of the titrant was determined three times by titration of the NaOH solution with 0.1 M aq. HCl.
Kinetic modelling
Kinetic modelling was conducted with Python, using Jupyter notebooks and the LMFIT, NumPy, Pandas, and SciPy libraries [75,76,77,78,79,80]. Experiments employing Glc, Fru, Man, and Allu as substrates at a starting concentration of 5 wt% or 10 wt% and different pH values (9.5, 10.5, or 11.5) as well as temperatures in the range of 50–90 °C were combined to one dataset and the modelling was performed. Only datapoints after the induction period and with a concentration > 0 mM were considered in kinetic modelling. To allow for simultaneous modelling at different reaction temperatures and account for the exact reaction temperature in each experiment, the Arrhenius equation (Eq. S14) was employed to calculate the reaction rate constants at different temperatures. During kinetic modelling, the ordinary differential equations for the reaction rates (Eqs. 9–12) were solved for the reaction times of the samples using the “odeint” command from SciPy. This was performed based on the normalized starting concentration of the hexoses (Eq. S6) and was used to estimate the reaction rate constants and activation energies. The temperatures measured in the reaction vessel at the end of each experiment as well as the average pH values measured in the reaction flask during the experiment were used in the modelling. Standard deviations of the optimized parameters were obtained in the kinetic modelling. The reference temperature was set to 70 °C for all experiments. This resulted in simulated concentrations for the four hexoses. Consequently, the difference between the simulated concentration and the measured concentration was minimized by variation of the reaction rate constants as well as the activation energies for each transformation using the “SLSQP” algorithm. Fit reports, containing the starting estimates and boundaries can be retrieved from the Supplementary Information.
Results and discussion
Yield and selectivity of the isomerization
We performed isomerization of Glc, Fru, and Man as substrates using aqueous NaOH at constant pH value as catalyst. Fig. 1 presents the selectivity-conversion plots of the isomerization starting from Glc, Fru, and Man at different temperatures. Overall, the selectivity decreases with increasing conversion, which can be attributed to the decomposition of monosaccharides into acidic side-products such as saccharinic acids, lactic acid, glyceric acid, and oligomeric side products [81, 82]. Since the decomposition products were removed before gas chromatographic analysis, the mass balance decreases over the course of the reaction (Figs. S1–S3). As shown in Fig. 1, the reaction of Glc to Fru, especially at low conversions, exhibits the highest selectivity compared to other isomerization reactions. In addition, co-formation of Allu based on Fru took place. This observation is in good agreement with the reaction network proposed by de Bruijn, suggesting a formation of Allu from Fru via a 2,3-enediol intermediate [81]. It can be seen from Fig. 1 that the selectivities starting from Man towards Glc and Fru are similar, but slightly higher towards Fru.
Selectivity for different substrates at various temperatures. “Glc → Fru” designates, for example, selectivity for Fru upon Glc isomerization. Reaction conditions: 150 mL of a 10 wt% aqueous hexose solution were placed into an oil bath heated to the given temperature; pH 10.5 was maintained by a periodic addition of NaOH solution using an auto titrator, stirring at 400 rpm
Selectivity-conversion plots for the isomerization reactions do not significantly depend either on temperature (50–70 °C, Fig. 1), or pH value (9.5–11.5, Fig. S4), or initial substrate concentration (2–10 wt%, Fig. S5)
Noteworthy, the presented datapoints correspond to the reaction rate after the induction time. An induction time is typical of the isomerization catalyzed by bases [14, 67, 83], which refers to a period, when the steady-state concentration of the enediol(s) has not been reached yet. The isomerization selectivities for the induction time are typically lower [14]. In some experiments, we also observed induction time which duration significantly depended on temperature, pH value, and substrate. Increase of pH values or temperatures resulted in reduced induction time. Induction times for isomerization of Glc and Fru were in range of 0 to 60 min. Induction times for Man isomerization were longer, up to two hours. It should be noted, however, that for most experiments no induction time was observed. This is due to the induction time being over before the initial addition of NaOH to the solution at reaction temperature was completed, which usually lasted for up to five minutes.
From practical point of view, it is interesting to compare the obtained yields in the presence of NaOH as catalyst with other soluble or solid base catalysts. The highest Fru yield obtained herein was 31% at a conversion of 50% at pH 10.5, 61 °C, and initial Glc concentration 10 wt% (Table S1). This result outperforms previously reported 25% [46] or 18% [15] yields obtained with NaOH as catalyst.
Comparable or slightly higher maximum yields of 19–41% were reported for soluble amines and nitrogen-containing solid bases [84, 85]. A similar trend can be seen for alkaline earth metal-containing catalysts, resulting in yields from 21 to 33% [14, 84], as well as for zeolites (YFru = 17–35%) and hydrotalcites (YFru = 25–42%) [84]. Catalytic properties of an anion exchange resin (YFru = 20%) [46] are outperformed by the catalytic system used in this work. Man was detected in yields up to 7% upon isomerization of Glc, which is in good agreement with literature data [11].
The backward reaction, isomerization of Fru to Glc, gave rise to Glc with the maximum yield of 30% obtained at a conversion of 68% at pH 10.5, 60 °C, and initial Fru concentration of 10 wt% (Table S2). The maximum Glc yield obtained in this work is comparable to the maximum yield of 28% reported for isomerization of Fru in presence of triazabicyclodecene (TBD) and higher than that of TBD grafted to a magnetically separable backbone (20%) [54]. Noteworthy, amine-based catalysts are often limited to low yields of up to 12% for conversions below 40% [45, 53, 54]. In this study, Man and Allu were formed starting from Fru in similar yields of up to 7%.
Upon isomerization of Man, the maximum yield of Fru (22%) was achieved at a conversion of 55% at pH 10.5, 65 °C, and substrate concentration 10 wt% (Table S3). The highest yield of Glc (19%) was recorded at a higher conversion of 72% under the same reaction conditions.
Kinetics of the reaction
Reaction network and kinetic model
Scheme 3 shows the reaction network used for modeling. It includes interconversion of four monosaccharides: Glc, Fru, Man, and Allu, as well as their decomposition products.
Applying quasi-equilibrium considerations discussed in Sect. 1.2, we obtained the following differential equations for the hexose consumption in the chemical reactions:
Here, apparent rate constants contain pH-independent (kA, k-A, kB, k-B, kC, k-C, kD, k-D, kE, kF, kG, kH) as well as pH-dependent (\(\frac{{K}_{\mathrm{Glc}}\left[{\mathrm{OH}}^{-}\right]}{1+{K}_{\mathrm{Glc}}\left[{\mathrm{OH}}^{-}\right]}, \frac{{K}_{\mathrm{Fru}}\left[{\mathrm{OH}}^{-}\right]}{1+{K}_{\mathrm{Fru}}\left[{\mathrm{OH}}^{-}\right]}, \frac{{K}_{\mathrm{Man}}\left[{\mathrm{OH}}^{-}\right]}{1+{K}_{\mathrm{Man}}\left[{\mathrm{OH}}^{-}\right]}, \mathrm{and} \frac{{K}_{\mathrm{Allu}}\left[{\mathrm{OH}}^{-}\right]}{1+{K}_{\mathrm{Allu}}\left[{\mathrm{OH}}^{-}\right]}\)) terms.
The reaction was performed in a semi-batch setup with varying volume due to the addition of aqueous NaOH as well as removal of samples from the reaction mixture. Thus, the reaction-time dependent volume of the reactor was described by fitting a quartic function to the experimental volume-time values (Eqs. S15–S16). The varying volume for the transformation of the hexose Hex was then accounted for according to Eq. 13.
Ionization constants of hexoses
The ordinary differential equations of the kinetic model (Eq. 9–12) contain the ionization constants of hexoses (\({K}_{\mathrm{Glc}}\), \({K}_{\mathrm{Fru}}\), \({K}_{\mathrm{Man}}\), and \({K}_{\mathrm{Allu}}\)). The values for \({K}_{\mathrm{Glc}}\), \({K}_{\mathrm{Fru}}\), and \({K}_{\mathrm{Man}}\) have already been reported, though some inconsistency in the literature data is present (Tables S4–S6) [68, 72, 86, 87]. According to our knowledge, the value for \({K}_{\mathrm{Allu}}\) is not available. To allow for a comparable dataset and considering the inconsistency between the various literature values [68, 72, 86, 87], we determined the ionization constants and standard reaction enthalpies by means of potentiometric titration at different temperatures for the four hexoses via their pKa values. Hence, pKa and pKw values were estimated for each titration simultaneously from experimental titration data using Bjerrum function plots, also known as difference plots (Fig. S7) [88]. The determined dissociation (\({K}_{\mathrm{a Hex}}\)) and ionization \({K}_{\mathrm{Hex}}\) constants for the saccharides as well as \(\Delta {H}^{\ominus }\) are listed in Table 1.
As seen from Table 1, the ionization constants of hexoses increase in the order: Glc < Fru < Man < Allu. This trend for Glc, Fru, and Man can also be observed for the data obtained by Izatt et al. [86], Christensen et al. [87], and de Wilt et al. [72] (Tables S4–S6). The pKa value determined for Allu was 11.81. In general, the resulting values for the ionization constants are in good agreement with literature data for \({K}_{\mathrm{Glc}}\) (31.0–52.2) [72, 86, 87] and \({K}_{\mathrm{Fru}}\) (49.0–53.3) [72, 86]. However, \({K}_{\mathrm{Fru}}\) was lower than the values reported by Christensen et al. (67.8–92.8) [87]. It should be noted though that the previously reported values for the ionization constants also deviate depending on the titration conditions, such as hexose concentration, titration temperature, ionic strength, and determination method [72, 86, 87]. The value of \({K}_{\mathrm{Man}}\) obtained in this work is lower than the values reported by Izatt et al. and Christensen et al., who obtained \({K}_{\mathrm{Man}}=\) 83.0–85.0 [86, 87]. \(\Delta {H}^{\ominus }\) was similar for all the hexoses (− 20.1 to − 28.5 kJ mol−1). The \(\Delta {H}^{\ominus }\) values determined in this work are in reasonable agreement with literature values (− 17.0 to − 25.6 kJ mol−1) [72, 86, 87].
Kinetic simulation
Based on the reaction network shown in Scheme 3 and using the determined ionization constants of the hexoses KHex, we performed kinetic simulations. Experimental data obtained for the pH values 9.5, 10.5, and 11.5 for the initial concentrations of substrates of 5 and 10 wt%, and for the temperature range of 50–90 °C were used.
All data were modelled simultaneously. The concentrations of hydroxide ions \(\left[{\mathrm{OH}}^{-}\right]\) were estimated from the pH values as well as the temperature-corrected pKw values, as reported in Eq. S9 [89]. Exemplary concentration–time plots for the isomerization starting from each hexose, which show the experimental as well as the fitted values, are presented in Fig. 2. Concentration–time plots including all the conducted experiments can be found in (Figs. S11–S14). As seen from the parity plot (Fig. S15), the experimental and the simulated values are in good agreement for all four hexoses, only presenting a random scatter around the ideal straight. Therefore, it can be concluded that the used kinetic model is applicable within the employed reaction conditions.
Experimental (exp) and fitted (fit) concentration–time plots of the alkaline isomerization starting from (left to right) Glc, Fru, Man, and Allu. Concentrations are normalized. Reaction conditions: 150 mL of a 10 wt% aqueous hexose solution were heated up to the indicated temperature in an oil bath; the pH value was maintained by a periodic addition of NaOH solution using an auto titrator, stirring at 400 rpm
Tables 2 and 3 list the resulting reaction rate constants at a reference temperature of 70 °C. For comparison with literature data (Table S8), the reaction rate constants adjusted to the temperatures employed in studies by de Bruijn et al. and Kooyman et al. [68] are provided in Table S9. Overall, the reaction rate constants obtained in this study are in good agreement with the previously reported ones [68, 73].
However, there are two disagreements of our results with the data of de Bruijn et al. [73]. First, our kinetic model suggests a somewhat higher rate constant for Glc− degradation (kE) than for Fru− (kF). This finding is unexpected, as previous studies by de Bruijn et al. [73], Marianou et al. [38], and de Wit et al. [67] reported the decomposition of Fru to be faster than the decomposition of Glc. Second, the reaction rate constants of decomposition reported by de Bruijn et al. are in general lower than the ones obtained in this work. These two deviations from previous studies are most likely interconnected and may result from the difference in employed reaction conditions. The study by de Bruijn et al. was carried out under an atmosphere of nitrogen, hindering decomposition via oxidation reactions [72, 73]. Our experiments were performed in air. Moreover, de Bruijn et al. used a lower substrate concentration of 0.025 M compared to the higher concentrations of 0.3–0.6 M employed in this work. Furthermore, on a molecular level, decomposition is accepted to occur via the 1,2 and 2,3 enediols. Thus, decomposition of Fru may be treated to occur partly through Allu, Glc and Man in kinetic modelling, resulting in an enhanced decomposition for the other hexoses. It is, however, possible to compare the sum of the decomposition rates for the aldoses at 70 °C (1.201 min−1) with the one of the ketoses (1.824 min−1). This data further supports a lower stability of ketoses compared to aldoses [67, 73].
Table 4 and Table 5 present the activation energies obtained by the fit. The activation energy for the isomerization of Glc− into Fru− Ea, A (113 ± 5 kJ mol−1) is higher than for the backward reaction Ea, −A (93 ± 6 kJ mol−1). This is in excellent agreement with the results obtained by de Wit et al. [67]. A slightly lower difference in activation energies was observed by Vourinen et al. for reactions in ethanol–water mixtures [90]. Despite using a similar kinetic model, we cannot confirm results of Kooyman et al. reporting the same activation energy for both reactions [68]. Our results are in reasonable agreement with the apparent activation energy determined for the isomerization in the presence of a phosphate buffer as catalytic system [91] (Table S10). Activation energies for the isomerization reactions are in the range of 50 and 196 kJ mol−1, which is in a larger range than the activation energies reported for the isomerization and epimerization of pentoses (107–146 kJ mol−1) [83] as well as previous reports on the aldose-ketose isomerization over solid catalysts (99 kJ mol−1) [10]. Furthermore, activation energies for decomposition (135 and 170 kJ mol−1) are comparable to the ones reported for decomposition of pentoses (87–206 kJ mol−1) [83].
The apparent equilibrium constants for the isomerization reactions as presented Eqs. S19–S20. For the Glc–Fru isomerization, a value of KA = 1.10 at 70 °C was obtained. This value is in good agreement with the ones reported by de Bruijn et al. (KA = 1.22–1.28) [73] at a slightly higher temperature of 78 °C, and Marianou et al. for catalysis by MgO at 90 °C (KA = 1.09) [38]. Our results are also in good agreement with the data reported by Tewari and Goldberg in their investigation of the biocatalytic isomerization [92] (Eq. S18). It should be noted that the calculated KA, as expected, increases with the temperature [92] albeit somewhat quicker than theoretically expected (Fig. S16). The equilibrium constants for all isomerization reactions are presented in Table 6. The resulting values for the other isomerization reactions are also similar to those obtained by de Bruijn et al. at a higher temperature of 78 °C (KB = 0.52–0.56, KC = 2.55–2.67, KD = 0.31) [73].
Model validation
To verify the applicability of the developed kinetic model and the parameters determined in this work, isomerization reactions were performed at a lower reaction temperature of 40 °C and substrate concentrations of 2 and 20 wt%. These experimental data were not included in the dataset of the kinetic modelling. Consequently, the concentrations were simulated for the reaction temperature and pH value of the experiments, using the parameters obtained in the kinetic modelling. An example of a plot for the model validation is presented in Fig. 3. Model validation for other experiments can be found in Figs. S17–S28.
Concentration–time plot (right) and parity plot (left) of the alkaline isomerization starting from Glc. Concentrations are normalized. Reaction conditions: 150 mL of a 10 wt% aqueous Glc solution were heated up to 38 °C in an oil bath; the pH 11.5 was maintained by a periodic addition of 3 M NaOH solution using an auto titrator, stirring at 400 rpm
Consequently, the kinetic model can be transferred to experiments at a lower reaction temperature and different concentrations reasonably well. Moreover, the model can be applied to simulate kinetic data for catalysis by SrO and Ba(OH)2 [14] as long as the pH value remains constant (ΔpH ≤ 0.3) (Fig. S29). Thus, the kinetic model obtained in this work is applicable for isomerization reactions at a constant pH value in the pH range of 9.5 to 11.5, temperature from 40 to 90 °C and a substrate concentration between 2 and 20 wt%.
Conclusion
In this work, we investigated the isomerization of Glc in aqueous NaOH as the simplest base catalyst enabling insight into the reaction kinetics. No significant influence of the temperature, substrate concentration, or the pH value on the conversion-selectivity plots was observed at the applied reaction conditions. The maximum Fru yield of 31% was obtained at Glc conversion of 50%, corresponding to selectivity of 60%. This outcome is comparable to the yields achieved with more complex catalysts.
We developed a kinetic model applicable in the pH range of 9.5 to 11.5, and between 40 and 90 °C for substrate concentrations of 2–20 wt%. Validity of the model was supported by the simulation experiments as well as parity plots. Calculated thermodynamic equilibrium constants for Glc–Fru isomerization were in good agreement with the literature data. The model enables calculation of reaction rate constants independent of the hydroxide-ion concentration and includes ionization constants of Glc, Fru, Man and Allu, which were determined by means of potentiometric titration. The activation energies for all the isomerization steps as well as for the decomposition reactions were determined. NaOH presents a useful benchmark system for comparison with more complex soluble and solid base catalysts, as shown here for alkaline earth metal (hydr)oxides. The obtained kinetic and thermodynamic data can be used to compare catalytic performance of various bases under different conditions.
Change history
03 November 2022
Missing Open Access funding information has been added in the Funding Note.
References
Hanover LM, White JS (1993) Manufacturing, composition, and applications of fructose. Am J Clin Nutr 58:724S-732S. https://doi.org/10.1093/ajcn/58.5.724S
Parker K, Salas M, Nwosu VC (2010) High fructose corn syrup: production, uses and public health concerns. Biotechnol Mol Biol Rev 5:71–78
Delidovich I, Palkovits R (2016) Catalytic isomerization of biomass-derived aldoses: a review. Chemsuschem 9:547–561. https://doi.org/10.1002/cssc.201501577
Delidovich I, Leonhard K, Palkovits R (2014) Cellulose and hemicellulose valorisation: an integrated challenge of catalysis and reaction engineering. Energy Environ Sci 7:2803–2830. https://doi.org/10.1039/C4EE01067A
Li H, Yang S, Saravanamurugan S, Riisager A (2017) Glucose isomerization by enzymes and chemo-catalysts: status and current advances. ACS Catal 7:3010–3029. https://doi.org/10.1021/acscatal.6b03625
Angyal SJ (2001). In: Stütz AE (ed) Glycoscience: epimerisation, isomerisation and rearrangement reactions of carbohydrates. Springer, Berlin, pp 1–14
Häsler H, Stütz AE (2001). In: Stütz AE (ed) Glycoscience: Epimerisation, isomerisation and rearrangement reactions of carbohydrates. Springer, Berlin, pp 77–114
Moreau C, Durand R, Roux A, Tichit D (2000) Isomerization of glucose into fructose in the presence of cation-exchanged zeolites and hydrotalcites. Appl Catal A 193:257–264. https://doi.org/10.1016/S0926-860X(99)00435-4
Souzanchi S, Nazari L, Rao KTV, Yuan Z, Tan Z, Xu CC (2019) Catalytic isomerization of glucose to fructose using heterogeneous solid Base catalysts in a continuous-flow tubular reactor: catalyst screening study. Catal Today 319:76–83. https://doi.org/10.1016/j.cattod.2018.03.056
Murzin DY, Murzina EV, Aho A, Kazakova MA, Selyutin AG, Kubicka D, Kuznetsov VL, Simakova IL (2017) Aldose to ketose interconversion: galactose and arabinose isomerization over heterogeneous catalysts. Catal Sci Technol 7:5321–5331. https://doi.org/10.1039/C7CY00281E
Marianou AA, Michailof CM, Pineda A, Iliopoulou EF, Triantafyllidis KS, Lappas AA (2016) Glucose to fructose isomerization in aqueous media over homogeneous and heterogeneous catalysts. ChemCatChem 8:1100–1110. https://doi.org/10.1002/cctc.201501203
Fu J, Shen F, Liu X, Qi X (2021) Synthesis of MgO-doped ordered mesoporous carbons by Mg2+-tannin coordination for efficient isomerization of glucose to fructose. Green Energy Environ. https://doi.org/10.1016/j.gee.2021.11.010
Chen SS, Cao Y, Tsang DC, Tessonnier J-P, Shang J, Hou D, Shen Z, Zhang S, Ok YS, Wu KC-W (2020) Effective dispersion of MgO nanostructure on biochar support as a basic catalyst for glucose isomerization. ACS Sustain Chem Eng 8:6990–7001. https://doi.org/10.1021/acssuschemeng.0c00278
Drabo P, Fischer M, Toussaint V, Flecken F, Palkovits R, Delidovich I (2021) What are the catalytically active species for aqueous-phase isomerization of D-glucose into D-fructose in the presence of alkaline earth metal (hydr)oxides? J Catal 402:315–324. https://doi.org/10.1016/j.jcat.2021.08.036
Delidovich I, Palkovits R (2014) Catalytic activity and stability of hydrophobic Mg–Al hydrotalcites in the continuous aqueous-phase isomerization of glucose into fructose. Catal Sci Technol 4:4322–4329. https://doi.org/10.1039/C4CY00776J
Delidovich I, Palkovits R (2015) Structure–performance correlations of Mg–Al hydrotalcite catalysts for the isomerization of glucose into fructose. J Catal 327:1–9. https://doi.org/10.1016/j.jcat.2015.04.012
Lecomte J, Finiels A, Moreau C (2002) Kinetic study of the isomerization of glucose into fructose in the presence of anion-modified hydrotalcites. Starch-Stärke 54:75–79. https://doi.org/10.1002/1521-379X(200202)54:2%3C75::AID-STAR75%3E3.0.CO;2-F
Lee G, Jeong Y, Takagaki A, Jung JC (2014) Sonication assisted rehydration of hydrotalcite catalyst for isomerization of glucose to fructose. J Mol Catal A: Chem 393:289–295. https://doi.org/10.1016/j.molcata.2014.06.019
Yu S, Kim E, Park S, Song IK, Jung JC (2012) Isomerization of glucose into fructose over Mg–Al hydrotalcite catalysts. Catal Commun 29:63–67. https://doi.org/10.1016/j.catcom.2012.09.015
Yabushita M, Shibayama N, Nakajima K, Fukuoka A (2019) Selective glucose-to-fructose isomerization in ethanol catalyzed by hydrotalcites. ACS Catal 9:2101–2109. https://doi.org/10.1021/acscatal.8b05145
Upare PP, Chamas A, Lee JH, Kim JC, Kwak SK, Hwang YK, Hwang DW (2019) Highly efficient hydrotalcite/1-butanol catalytic system for the production of the high-yield fructose crystal from glucose. ACS Catal 10:1388–1396. https://doi.org/10.1021/acscatal.9b01650
Iris K, Hanif A, Tsang DC, Shang J, Su Z, Song H, Ok YS, Poon CS (2020) Tuneable functionalities in layered double hydroxide catalysts for thermochemical conversion of biomass-derived glucose to fructose. Chem Eng J 383:122914. https://doi.org/10.1016/j.cej.2019.122914
An S, Kwon D, Cho J, Jung JC (2020) Effect of the solvent on the basic properties of Mg–Al hydrotalcite catalysts for glucose isomerization. Catalysts 10:1236. https://doi.org/10.3390/catal10111236
Steinbach D, Klier A, Kruse A, Sauer J, Wild S, Zanker M (2020) Isomerization of glucose to fructose in hydrolysates from lignocellulosic biomass using hydrotalcite. Processes. https://doi.org/10.3390/pr8060644
Graça I, Bacariza M, Chadwick D (2018) Glucose isomerisation into fructose over Mg-impregnated Na-zeolites: influence of zeolite structure. Microporous Mesoporous Mater 255:130–139. https://doi.org/10.1016/j.micromeso.2017.07.015
Graça I, Iruretagoyena D, Chadwick D (2017) Glucose isomerisation into fructose over magnesium-impregnated NaY zeolite catalysts. Appl Catal B 206:434–443. https://doi.org/10.1016/j.apcatb.2017.01.037
Graça I, Bacariza M, Fernandes A, Chadwick D (2018) Desilicated NaY zeolites impregnated with magnesium as catalysts for glucose isomerisation into fructose. Appl Catal B 224:660–670. https://doi.org/10.1016/j.apcatb.2017.11.009
Antunes MM, Fernandes A, Falcão D, Pillinger M, Ribeiro F, Valente AA (2020) Optimized preparation and regeneration of MFI type base catalysts for d-glucose isomerization in water. Catal Sci Technol 10:3232–3246. https://doi.org/10.1039/D0CY00188K
Antunes MM, Falcão D, Fernandes A, Ribeiro F, Pillinger M, Rocha J, Valente AA (2021) Catalytic isomerization of d-glucose to d-fructose over BEA base zeotypes using different energy supply methods. Catal Today 362:162–174. https://doi.org/10.1016/j.cattod.2020.03.024
Li B, Li L, Zhang Q, Weng W, Wan H (2017) Attapulgite as natural catalyst for glucose isomerization to fructose in water. Catal Commun 99:20–24. https://doi.org/10.1016/j.catcom.2017.05.011
Li B, Jiang H, Zhao X, Pei Z, Zhang Q (2020) Enhanced conversion of glucose to fructose over naturalattapulgite catalyst promoted by CeO2 in water. ChemistrySelect 5:14971–14977. https://doi.org/10.1002/slct.202004064
Gao D-M, Shen Y-B, Zhao B, Liu Q, Nakanishi K, Chen J, Kanamori K, Wu H, He Z, Zeng M (2019) Macroporous niobium phosphate-supported magnesia catalysts for isomerization of glucose-to-fructose. ACS Sustain Chem Eng 7:8512–8521. https://doi.org/10.1021/acssuschemeng.9b00292
Laiq Ur Rehman M, Hou Q, Bai X, Nie Y, Qian H, Xia T, Lai R, Yu G, Ju M (2022) Regulating the alkalinity of carbon nitride by magnesium doping to boost the selective isomerization of glucose to fructose. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.1c07832
Antunes MM, Fernandes A, Ribeiro MF, Lin Z, Valente AA (2020) Modified versions of AM-4 for the aqueous phase isomerization of aldo-saccharides. Eur J Inorg Chem 2020:1579–1588. https://doi.org/10.1002/ejic.202000038
Kitajima H, Higashino Y, Matsuda S, Zhong H, Watanabe M, Aida TM, Smith RL Jr (2016) Isomerization of glucose at hydrothermal condition with TiO2, ZrO2, CaO-doped ZrO2 or TiO2-doped ZrO2. Catal Today 274:67–72. https://doi.org/10.1016/j.cattod.2016.01.049
Rabee AI, Le SD, Nishimura S (2020) MgO-ZrO2 mixed oxides as effective and reusable base catalysts for glucose isomerization into fructose in aqueous media. Chem Asian J 15:294–300. https://doi.org/10.1002/asia.201901534
Ventura M, Cecilia JA, Rodríguez-Castellón E, Domine ME (2020) Tuning Ca–Al-based catalysts’ composition to isomerize or epimerize glucose and other sugars. Green Chem 22:1393–1405. https://doi.org/10.1039/C9GC02823D
Marianou AA, Michailof CM, Ipsakis DK, Karakoulia SA, Kalogiannis KG, Yiannoulakis H, Triantafyllidis KS, Lappas AA (2018) Isomerization of glucose into fructose over natural and synthetic MgO catalysts. ACS Sustain Chem Eng 6:16459–16470. https://doi.org/10.1021/acssuschemeng.8b03570
Shen F, Fu J, Zhang X, Qi X (2019) Crab shell-derived lotus rootlike porous carbon for high efficiency isomerization of glucose to fructose under mild conditions. ACS Sustain Chem Eng 7:4466–4472. https://doi.org/10.1021/acssuschemeng.8b06512
Ohyama J, Zhang Y, Ito J, Satsuma A (2017) Glucose isomerization using alkali metal and alkaline earth metal titanates. ChemCatChem 9:2864–2868. https://doi.org/10.1002/cctc.201700068
Liu C, Carraher JM, Swedberg JL, Herndon CR, Fleitman CN, Tessonnier J-P (2014) Selective base-catalyzed isomerization of glucose to fructose. ACS Catal 4:4295–4298. https://doi.org/10.1021/cs501197w
Carraher JM, Fleitman CN, Tessonnier J-P (2015) Kinetic and mechanistic study of glucose isomerization using homogeneous organic brønsted base catalysts in water. ACS Catal 5:3162–3173. https://doi.org/10.1021/acscatal.5b00316
Deshpande N, Cho EH, Spanos AP, Lin L-C, Brunelli NA (2019) Tuning molecular structure of tertiary amine catalysts for glucose isomerization. J Catal 372:119–127. https://doi.org/10.1016/j.jcat.2019.02.025
Yang Q, Runge T (2016) Polyethylenimines as homogeneous and heterogeneous catalysts for glucose isomerization. ACS Sustain Chem Eng 4:6951–6961. https://doi.org/10.1021/acssuschemeng.6b01880
Yang Q, Sherbahn M, Runge T (2016) Basic amino acids as green catalysts for isomerization of glucose to fructose in water. ACS Sustain Chem Eng 4:3526–3534. https://doi.org/10.1021/acssuschemeng.6b00587
Chen SS, Tsang DCW, Tessonnier J-P (2020) Comparative investigation of homogeneous and heterogeneous Brønsted base catalysts for the isomerization of glucose to fructose in aqueous media. Appl Catal B 261:118126. https://doi.org/10.1016/j.apcatb.2019.118126
Chen SS, Carraher JM, Tuci G, Rossin A, Raman CA, Luconi L, Tsang DC, Giambastiani G, Tessonnier J-P (2019) Engineered nitrogen-decorated carbon networks for the metal-free catalytic isomerization of glucose to fructose. ACS Sustain Chem Eng 7:16959–16963. https://doi.org/10.1021/acssuschemeng.9b04067
Chen SS, Yu IK, Cho D-W, Song H, Tsang DC, Tessonnier J-P, Ok YS, Poon CS (2018) Selective glucose isomerization to fructose via a nitrogen-doped solid base catalyst derived from spent coffee grounds. ACS Sustain Chem Eng 6:16113–16120. https://doi.org/10.1021/acssuschemeng.8b02752
Li X, Zhang X, Li H, Long J (2019) Glucose isomerizes to fructose catalyzed by the eco-friendly and biodegradable ionic liquids. ChemistrySelect 4:13731–13735. https://doi.org/10.1002/slct.201904192
Zhang X, Li H, Li X, Liu Y, Li X, Guan J, Long J (2019) Glucose aqueous isomerization catalyzed by basic ionic liquids. ACS Sustain Chem Eng 7:13247–13256. https://doi.org/10.1021/acssuschemeng.9b02495
Zhang N, Meng XG, Wu YY, Song HJ, Huang H, Wang F, Lv J (2019) Highly selective isomerization of glucose into fructose catalyzed by a mimic glucose isomerase. ChemCatChem 11:2355–2361. https://doi.org/10.1002/cctc.201900143
Deshpande N, Pattanaik L, Whitaker MR, Yang C-T, Lin L-C, Brunelli NA (2017) Selectively converting glucose to fructose using immobilized tertiary amines. J Catal 353:205–210. https://doi.org/10.1016/j.jcat.2017.07.021
Yang Q, Lan W, Runge T (2016) Salt-promoted glucose aqueous isomerization catalyzed by heterogeneous organic base. ACS Sustain Chem Eng 4:4850–4858. https://doi.org/10.1021/acssuschemeng.6b01132
Yang Q, Zhou S, Runge T (2015) Magnetically separable base catalysts for isomerization of glucose to fructose. J Catal 330:474–484. https://doi.org/10.1016/j.jcat.2015.08.008
Wang Y, Wang J, Zhang Y, Song F, Xie Y, Wang M, Cui H, Yi W (2020) N-doped carbon materials as heterogeneous catalysts for high efficiency isomerization glucose to fructose in aqueous media. Catal Lett 150:493–504. https://doi.org/10.1007/s10562-019-03020-1
Guo Z, Pedersen CM, Wang P, Ma M, Zhao Y, Qiao Y, Wang Y (2021) D-Glucose isomerization with PAMAM dendrimers as environmentally friendly catalysts. J Agric Food Chem 69:5105–5112. https://doi.org/10.1021/acs.jafc.1c01088
Lima S, Dias AS, Lin Z, Brandão P, Ferreira P, Pillinger M, Rocha J, Calvino-Casilda V, Valente AA (2008) Isomerization of D-glucose to D-fructose over metallosilicate solid bases. Appl Catal A 339:21–27. https://doi.org/10.1016/j.apcata.2007.12.030
Otomo R, Fujimoto M, Nagao M, Kamiya Y (2019) Ammonia-treated metal oxides as base catalysts for selective isomerization of glucose in water. Mol Catal 475:110479. https://doi.org/10.1016/j.mcat.2019.110479
Son PA, Nishimura S, Ebitani K (2014) Preparation of zirconium carbonate as water-tolerant solid base catalyst for glucose isomerization and one-pot synthesis of levulinic acid with solid acid catalyst. Reac Kinet Mech Cat 111:183–197. https://doi.org/10.1007/s11144-013-0642-6
Watanabe M, Aizawa Y, Iida T, Nishimura R, Inomata H (2005) Catalytic glucose and fructose conversion with TiO2 and ZrO2 in water at 473 K: relationship between reactivity and acid–base property determined by TPD measurement. Appl Catal A 295:150–156. https://doi.org/10.1016/j.apcata.2005.08.007
Souza RO, Fabiano DP, Feche C, Rataboul F, Cardoso D, Essayem N (2012) Glucose–fructose isomerisation promoted by basic hybrid catalysts. Catal Today 195:114–119. https://doi.org/10.1016/j.cattod.2012.05.046
Toussaint V, Delidovich I (2022) Revealing the contributions of homogeneous and heterogeneous catalysis for isomerization of D-glucose into D-fructose in the presence of low-soluble basic salts. Catal Sci Technol. https://doi.org/10.1039/D2CY00551D
Ventura M, Mazarío J, Domine ME (2022) Isomerization of glucose-to-fructose in water over a continuous flow reactor using Ca−Al mixed oxide as heterogeneous catalyst. ChemCatChem 14:e202101229. https://doi.org/10.1002/cctc.202101229
Moliner M, Román-Leshkov Y, Davis Mark E (2010) Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc Natl Acad Sci USA 107:6164–6168. https://doi.org/10.1073/pnas.1002358107
Lari GM, Dapsens PY, Scholz D, Mitchell S, Mondelli C, Pérez-Ramírez J (2016) Deactivation mechanisms of tin-zeolites in biomass conversions. Green Chem 18:1249–1260. https://doi.org/10.1039/C5GC02147B
Botti L, Kondrat SA, Navar R, Padovan D, Martinez-Espin JS, Meier S, Hammond C (2020) Solvent-activated hafnium-containing zeolites enable selective and continuous glucose-fructose isomerisation. Angew Chem Int Ed 59:20017–20023. https://doi.org/10.1002/anie.202006718
de Wit G, Kieboom APG, van Bekkum H (1979) Enolisation and isomerisation of monosaccharides in aqueous, alkaline solution. Carbohydr Res 74:157–175. https://doi.org/10.1016/S0008-6215(00)84773-4
Kooyman C, Vellenga K, De Wilt HGJ (1977) The isomerization of d-glucose into d-fructose in aqueous alkaline solutions. Carbohydr Res 54:33–44. https://doi.org/10.1016/S0008-6215(77)80003-7
de Wit G, Kieboom APG, van Bekkum H (1975) Ionization and mutarotation of hexoses in aqueous alkaline solution as studied by 13C-NMR spectroscopy. Tetrahedron Lett 16:3943–3946. https://doi.org/10.1016/S0040-4039(00)91260-7
Isbell HS, Frush HL, Wade CWR, Hunter CE (1969) Transformations of sugars in alkaline solutions. Carbohydr Res 9:163–175. https://doi.org/10.1016/S0008-6215(00)82132-1
Isbell HS, Linek K, Hepner KE Jr (1971) Transformations of sugars in alkaline solutions: Part II. Primary rates of enolization. Carbohydr Res 19:319–327. https://doi.org/10.1016/S0008-6215(00)86162-5
de Wilt HGJ, Lindhout I (1972) The oxidation of D-glucose and D-fructose with oxygen in aqueous, alkaline solutions: Part II. The overall kinetics. Carbohydr Res 23:333–341. https://doi.org/10.1016/S0008-6215(00)82703-2
de Bruijn JM, Kieboom APG, van Bekkum H (1987) Alkaline degradation of monosaccharides V: kinetics of the alkaline isomerization and degradation of monosaccharides. Recl Trav Chim Pays-Bas 106:35–43. https://doi.org/10.1002/recl.19871060201
Ekeberg D, Morgenlie S, Stenstrøm Y (2005) Isomerisation of aldoses in pyridine in the presence of aluminium oxide. Carbohydr Res 340:373–377. https://doi.org/10.1016/j.carres.2004.12.006
Matt Newville RO, Nelson A, Ingargiola A, Stensitzki T, Allan D, Fox A, Carter F, Michał, Pustakhod D, lneuhaus, Weigand S, Osborn R, Glenn, Deil C, Mark, Hansen ALR, Pasquevich G, Foks L, Zobrist N, Frost O, Beelen A, Stuermer, kwertyops, Polloreno A, Caldwell S, Almarza A, Persaud A, Gamari B, Maier BF (2021) lmfit/lmfit-py 1.0.2. zenodo. https://doi.org/10.5281/zenodo.451665
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, van der Walt SJ, Brett M, Wilson J, Millman KJ, Mayorov N, Nelson ARJ, Jones E, Kern R, Larson E, Carey CJ, Polat İ, Feng Y, Moore EW, VanderPlas J, Laxalde D, Perktold J, Cimrman R, Henriksen I, Quintero EA, Harris CR, Archibald AM, Ribeiro AH, Pedregosa F, van Mulbregt P, Vijaykumar A, Bardelli AP, Rothberg A, Hilboll A, Kloeckner A, Scopatz A, Lee A, Rokem A, Woods CN, Fulton C, Masson C, Häggström C, Fitzgerald C, Nicholson DA, Hagen DR, Pasechnik DV, Olivetti E, Martin E, Wieser E, Silva F, Lenders F, Wilhelm F, Young G, Price GA, Ingold G-L, Allen GE, Lee GR, Audren H, Probst I, Dietrich JP, Silterra J, Webber JT, Slavič J, Nothman J, Buchner J, Kulick J, Schönberger JL, de Miranda Cardoso JV, Reimer J, Harrington J, Rodríguez JLC, Nunez-Iglesias J, Kuczynski J, Tritz K, Thoma M, Newville M, Kümmerer M, Bolingbroke M, Tartre M, Pak M, Smith NJ, Nowaczyk N, Shebanov N, Pavlyk O, Brodtkorb PA, Lee P, McGibbon RT, Feldbauer R, Lewis S, Tygier S, Sievert S, Vigna S, Peterson S, More S, Pudlik T, Oshima T, Pingel TJ, Robitaille TP, Spura T, Jones TR, Cera T, Leslie T, Zito T, Krauss T, Upadhyay U, Halchenko YO, Vázquez-Baeza Y, SciPy C (2020) SciPy 1.0: fundamental algorithms for scientific computing in python. Nat Methods 17:261–272. https://doi.org/10.1038/s41592-019-0686-2
Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, Kern R, Picus M, Hoyer S, van Kerkwijk MH, Brett M, Haldane A, del Río JF, Wiebe M, Peterson P, Gérard-Marchant P, Sheppard K, Reddy T, Weckesser W, Abbasi H, Gohlke C, Oliphant TE (2020) Array programming with NumPy. Nature 585:357–362. https://doi.org/10.1038/s41586-020-2649-2
Perez F, Granger BE (2007) IPython: a system for interactive scientific computing. Comput Sci Eng 9:21–29. https://doi.org/10.1109/MCSE.2007.53
Hunter JD (2007) Matplotlib: a 2D graphics environment. Comput Sci Eng 9:90–95. https://doi.org/10.1109/MCSE.2007.55
McKinney W (2010) Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, pp 56–61. https://doi.org/10.25080/Majora-92bf1922-00a
de Bruijn JM, Kieboom APG, van Bekkum H (1987) Alkaline degradation of monosaccharides Part VII. A mechanistic picture. Starch Stärke 39:23–28. https://doi.org/10.1002/star.19870390107
Yang BY, Montgomery R (1996) Alkaline degradation of glucose: effect of initial concentration of reactants. Carbohydr Res 280:27–45. https://doi.org/10.1016/0008-6215(95)00294-4
Delidovich I, Gyngazova MS, Sánchez-Bastardo N, Wohland JP, Hoppe C, Drabo P (2018) Production of keto-pentoses via isomerization of aldo-pentoses catalyzed by phosphates and recovery of products by anionic extraction. Green Chem 20:724–734. https://doi.org/10.1039/C7GC03077K
Delidovich I (2021) Recent progress in base-catalyzed isomerization of D-glucose into D-fructose. Curr Opin Green Sustain Chem 27:100414. https://doi.org/10.1016/j.cogsc.2020.100414
Kumar S, Sharma S, Kansal SK, Elumalai S (2020) Efficient conversion of glucose into fructose via extraction-assisted isomerization catalyzed by endogenous polyamine spermine in the aqueous phase. ACS Omega 5:2406–2418. https://doi.org/10.1021/acsomega.9b03918
Izatt RM, Rytting JH, Hansen LD, Christensen JJ (1966) Thermodynamics of proton dissociation in dilute aqueous solution. V. An entropy titration study of adenosine, pentoses, hexoses, and related Compounds1a, b. J Am Chem Soc 88:2641–2645. https://doi.org/10.1021/ja00964a003
Christensen JJ, Rytting JH, Izatt RM (1970) Thermodynamics of proton dissociation in dilute aqueous solution. Part XV. Proton dissociation from several monosaccharides at 10 and 40 °C. J Chem Soc B. https://doi.org/10.1039/J29700001646
Kraft A (2003) The determination of the pKa of multiprotic, weak acids by analyzing potentiometric acid-base titration data with difference plots. J Chem Educ 80:554. https://doi.org/10.1021/ed080p554
Lemmon EW, Harvey AH (2017). In: Haynes WM, Lide DR, Bruno TJ (eds) CRC Handbook of Chemistry and Physics, 97th edn. Taylor & Francis Group, Boca Raton
Vuorinen T, Sjöström E (1982) Kinetics of alkali-catalyzed isomerization of D-glucose and D-fructose in ethanol-water solutions. Carbohydr Res 108:23–29. https://doi.org/10.1016/S0008-6215(00)81886-8
Delidovich I, Palkovits R (2016) Fructose production via extraction-assisted isomerization of glucose catalyzed by phosphates. Green Chem 18:5822–5830. https://doi.org/10.1039/C6GC01712F
Tewari YB, Goldberg RN (1984) Thermodynamics of the conversion of aqueous glucose to fructose. J Solut Chem 13:523–547. https://doi.org/10.1007/BF00647222
Acknowledgements
We thank Prof. Dr. Regina Palkovits for her valuable input and support. We thank Carina Frantzen, and Frederic Thilmany for their experimental contributions. Moreover, we thank Elke Biener, Hannelore Eschmann, Heike Fickers-Boltz, and Magda Kraszewski for conduction the gas chromatography measurements. We gratefully acknowledge funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under the project number 397970309. This work partly contributed to the Cluster of Excellence “The Fuel Science Center”, which is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy Exzellenzcluster 2186 “The Fuel Science Center” (ID: 390919832).
Funding
Open access funding provided by TU Wien (TUW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, M., Drabo, P. & Delidovich, I. Study of base-catalyzed isomerization of d-glucose with a focus on reaction kinetics. Reac Kinet Mech Cat 135, 2357–2377 (2022). https://doi.org/10.1007/s11144-022-02277-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02277-9