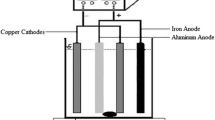
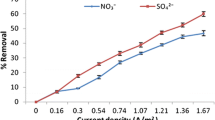
Abstract
In this study, the use of hydrogen peroxide increased the efficiency of electrocoagulation (EC) by 48%. The data on boron adsorption on the released aluminum in enhanced electrocoagulation (EEC) correspond to Langmuir (R2 = 0.59) and Freundlich (R2 = 0.68) isotherms. The data on the kinetics of boron removal by EEC were consistent with the Pseudo-first order kinetics kinetic model (R2 = 0.99). The rate of boron removal in the EEC is 1.85 times higher than that of the EC. The efficiency of EEC in the removal of boric acid under optimal conditions was more than 88%. Also, the cost of electricity consumption in optimal conditions of EEC is about 39% of EC costs. Therefore, the EEC can be used as an efficient and economical technique to treat boron-contaminated water at low concentrations (10 mg/L) and in neutral environments to meet drinking water standards.






Similar content being viewed by others
References
Han D, Hwang M, Kim IS (2017) Effect of boron rejection and recovery rate on a single-pass design of SWRO using hybrid membrane inter-stage design (HID) concept. Desalination 404(Supplement C):215–23. https://doi.org/10.1016/j.desal.2016.11.007
Shultz S, Freger V (2017) In situ modification of membrane elements for improved boron rejection in RO desalination. Desalination. https://doi.org/10.1016/j.desal.2017.08.021
Kürklü S, Velioğlu S, Ahunbay MG, Tantekin-Ersolmaz SB, Krantz WB (2017) A novel energy-efficient concurrent desalination and boron removal (CDBR) process. Desalination 423(Suuplement C):79–94. https://doi.org/10.1016/j.desal.2017.09.005
Lin J-Y, Mahasti NN, Huang Y-H (2021) Recent advances in adsorption and coagulation for boron removal from wastewater: a comprehensive review. J Hazard Mater 407:124401
Dolati M, Aghapour AA, Khorsandi H, Karimzade S (2017) Boron removal from aqueous solutions by electrocoagulation at low concentrations. J Environ Chem Eng 5(5):5150–5156. https://doi.org/10.1016/j.jece.2017.09.055
Gargouri B, Gargouri OD, Khmakhem I, Ammar S, Abdelhèdi R, Bouaziz M (2017) Chemical composition and direct electrochemical oxidation of table olive processing wastewater using high oxidation power anodes. Chemosphere 166:363–371. https://doi.org/10.1016/j.chemosphere.2016.09.080
Guan Z, Lv J, Bai P, Guo X (2016) Boron removal from aqueous solutions by adsorption—a review. Desalination 383(Supplement C):29–37. https://doi.org/10.1016/j.desal.2015.12.026
Wided B, Khawla M, Eya BK, Béchir H. Evaluation of boron removal by coagulation-flocculation and electrocoagulation. Int J Eng Res Technol: ESRSA Publications; 2014
Oladipo AA, Gazi M (2016) Efficient boron abstraction using honeycomb-like porous magnetic hybrids: assessment of techno-economic recovery of boric acid. J Environ Chem Eng 183:917–924. https://doi.org/10.1016/j.jenvman.2016.09.059
Yilmaz AE, Boncukcuoğlu R, Kocakerim MM, Yilmaz MT, Paluluoğlu C (2008) Boron removal from geothermal waters by electrocoagulation. J Hazard Mater 153(1–2):146–151. https://doi.org/10.1016/j.jhazmat.2007.08.030
Sayiner G, Kandemirli F, Dimoglo A (2008) Evaluation of boron removal by electrocoagulation using iron and aluminum electrodes. Desalination 230(1–3):205–212. https://doi.org/10.1016/j.desal.2007.10.020
Aghapour AA, Khorsandi H, Dehghani A, Karimzade S (2018) Preparation and characterization and application of activated alumina (AA) from alum sludge for the adsorption of fluoride from aqueous solutions: new approach to alum sludge recycling. Water Supply 18(5):1825–1831. https://doi.org/10.2166/ws.2018.006
Vasudevan S, Lakshmi J (2012) Electrochemical removal of boron from water: adsorption and thermodynamic studies. Can J Chem Eng 90(4):1017–1026
Organization WH (2009) Boron in drinking-water: background document for development of WHO guidelines for drinking-water quality No WHO/HSE/WSH/09.01/2. World Health Organization,
Bashir MJK, Lim JH, Abu Amr SS, Wong LP, Sim YL (2019) Post treatment of palm oil mill effluent using electro-coagulation-peroxidation (ECP) technique. J Clean Prod 208:716–727. https://doi.org/10.1016/j.jclepro.2018.10.073
Esfandyari Y, Mahdavi Y, Seyedsalehi M, Hoseini M, Safari GH, Ghozikali MG et al (2015) Degradation and biodegradability improvement of the olive mill wastewater by peroxi-electrocoagulation/electrooxidation-electroflotation process with bipolar aluminum electrodes. Environ Sci Pollut Res 22(8):6288–6297. https://doi.org/10.1007/s11356-014-3832-5
Gong C, Zhang Z, Zhang J, Li S (2017) The addition of hydrogen peroxide in the electrocoagulation treatment for improving toxic organic matters removal: a comparative study. Sep Sci Technol 52(8):1404–1411
Kenova TA, Vasil’eva IS, Kornienko VL (2016) Removal of thiocyanates and heavy metal ions from simulated wastewater solutions by electro- and peroxyelectrocoagulation. Russ J Appl Chem 89:1440–1446
Kürklü S, Velioğlu S, Ahunbay MG, Tantekin-Ersolmaz SB, Krantz WB (2017) A novel energy-efficient concurrent desalination and boron removal (CDBR) process. Desalination 423:79–94. https://doi.org/10.1016/j.desal.2017.09.005
Federation WE, Association A (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC, USA, p 21
You HJ, Han IS (2016) Effects of dissolved ions and natural organic matter on electrocoagulation of As (III) in groundwater. J Environ Chem Eng 4(1):1008–16
Zhou S, Bu L, Shi Z, Bi C, Yi Q (2016) A novel advanced oxidation process using iron electrodes and ozone in atrazine degradation: performance and mechanism. Chem Eng J 306:719–725
Aghapour AA, Khorsandi H, Dehghani A, Karimzade S (2018) Preparation and characterization and application of activated alumina (AA) from alum sludge for the adsorption of fluoride from aqueous solutions: new approach to alum sludge recycling. Water Sci Technol: Water Supply 18(5):1825–1831
Ebba M, Asaithambi P, Alemayehu E (2021) Investigation on operating parameters and cost using an electrocoagulation process for wastewater treatment. Appl Water Sci 11(11):175. https://doi.org/10.1007/s13201-021-01517-y
Lin J-Y, Shih Y-J, Chen P-Y, Huang Y-H (2016) Precipitation recovery of boron from aqueous solution by chemical oxo-precipitation at room temperature. Appl Energy 164:1052–1058. https://doi.org/10.1016/j.apenergy.2014.12.058
Shih Y-J, Liu C-H, Lan W-C, Huang Y-H (2014) A novel chemical oxo-precipitation (COP) process for efficient remediation of boron wastewater at room temperature. Chemosphere 111:232–237. https://doi.org/10.1016/j.chemosphere.2014.03.121
Song P, Yang Z, Zeng G, Yang X, Xu H, Wang L et al (2017) Electrocoagulation treatment of arsenic in wastewaters: a comprehensive review. Chem Eng J 317:707–725. https://doi.org/10.1016/j.cej.2017.02.086
Qiang Z, Chang J-H, Huang C-P (2002) Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions. Water Res 36(1):85–94. https://doi.org/10.1016/S0043-1354(01)00235-4
Holt PK, Barton GW, Mitchell CA (2005) The future for electrocoagulation as a localised water treatment technology. Chemosphere 59(3):355–367. https://doi.org/10.1016/j.chemosphere.2004.10.023
Nidheesh PV, Singh TSA (2017) Arsenic removal by electrocoagulation process: recent trends and removal mechanism. Chemosphere 181:418–432. https://doi.org/10.1016/j.chemosphere.2017.04.082
Ibarra-Taquez HN, GilPavas E, Blatchley Iii ER, Gómez-García M-Á, Dobrosz-Gómez I (2017) Integrated electrocoagulation-electrooxidation process for the treatment of soluble coffee effluent: optimization of COD degradation and operation time analysis. J Environ Chem Eng 200:530–538. https://doi.org/10.1016/j.jenvman.2017.05.095
Isa MH, Ezechi EH, Ahmed Z, Magram SF, Kutty SRM (2014) Boron removal by electrocoagulation and recovery. Water Res 51:113–123. https://doi.org/10.1016/j.watres.2013.12.024
Bektaş N, Öncel S, Akbulut HY, Dimoglo A (2004) Removal of boron by electrocoagulation. Environ Chem Lett 2(2):51–54
Edition FJWc (2011) Guidelines for drinking-water quality. 38(4):304–308.
Lente G (2018) Facts and alternative facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng. https://doi.org/10.1016/j.coche.2018.03.007
Acknowledgements
This paper is a part of the results of a master's thesis in Environmental Health Engineering with the research code 1394-0-34-1805. We want to express our gratitude to the Urmia University of Medical Sciences that financed the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghapour, A.A., Dolati, M. & Khorsandi, H. Boron removal using enhanced electrocoagulation (EEC) with hydrogen peroxide under natural conditions to prepare drinking water. Reac Kinet Mech Cat 135, 2073–2084 (2022). https://doi.org/10.1007/s11144-022-02246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02246-2