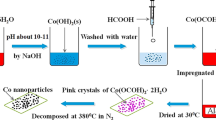
Abstract
In this study ceramic supported noble metal-free catalysts promoting the oxidation of CO were examined. In the course of our work several non-noble metal containing catalysts were prepared with different metal content by the well-known wet impregnation method and their catalytic activities were analyzed by gas chromatography (GC) experiments in CO oxidation reaction. In addition to GC measurements, X-ray diffraction, scanning electron microscopy, BET and X-ray photoelectron spectroscopy tests were also performed on our samples. During our work we found that cobalt-loaded silica-alumina-based ceramic supported catalyst proved to be the best in CO oxidation due to the high activity and durability with comparable activity with Pt-loaded counterpart.







Similar content being viewed by others
References
Tarín-Carrasco P, Im U, Geels C, Palacios-Pena L, Jimenez-Guerrero P (2021) Contribution of fine particulate matter to present and future premature mortality over Europe: a non-linear response. Environ Int. https://doi.org/10.1016/j.envint.2021.106517
Mattiuzzi C, Lippi G (2019) Worldwide epidemiology of carbon monoxide poisoning. Hum Exp Toxicol. https://doi.org/10.1177/0960327119891214
Janík M, Ublova M, Kucerova S, Hejna P (2017) Carbon monoxide-related fatalities: a 60-year single institution experience. J Forensic Leg Med. https://doi.org/10.1016/j.jflm.2017.04.002
Westberg K, Cohen N, Wilson KW (1971) Carbon monoxide: its role in photochemical smog formation. Science. https://doi.org/10.1126/science.171.3975.1013
Haszpra L, Ferenczi Z, Barcza Z (2019) Estimation of greenhouse gas emission factors based on observed covariance of CO2, CH4, N2O and CO mole fractions. Environ Sci Eur. https://doi.org/10.1186/s12302-019-0277-y
Gatla S, Aubert D, Agostini A, Mathon O, Pascarelli S, Lunkenbein T, Willinger MG, Kaper H (2016) Room-temperature CO oxidation catalyst: low temperature metal-support interaction between platinum nanoparticles and nanosized ceria. ACS Catal. https://doi.org/10.1021/acscatal.6b00677
Jansson J (2000) Low-temperature CO oxidation over Co3O4/Al2O3. J Catal. https://doi.org/10.1006/jcat.2000.2924
Royer S, Duprez D (2011) Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem. https://doi.org/10.1002/cctc.201000378
Dey S, Dhal GC, Mohan D, Prasad R (2018) The choice of precursors in the synthesizing of CuMnOx catalysts for maximizing CO oxidation. Int J Ind Chem. https://doi.org/10.1007/s40090-018-0150-7
Moreau F, Bond GC (2007) Preservation of the activity of supported gold catalysts for CO oxidation. Top Catal. https://doi.org/10.1007/s11244-007-0282-z
Michalak WD, Krier JM, Alayoglu S, Shin J, An K, Kyriakos K, Liu Z, Somorjai GA (2014) CO oxidation on PtSn nanoparticle catalysts occurs at the interface of Pt and Sn oxide domains formed under reaction conditions. J Catal. https://doi.org/10.1016/j.jcat.2014.01.005
Soliman NK (2019) Factors affecting CO oxidation reaction over nanosized materials: a review. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2018.12.012
Kim IH, Seo HO, Park EJ, Han SW, Kim YD (2017) Low temperature CO oxidation over iron oxide nanoparticles decorating internal structures of a mesoporous alumina. Sci Rep. https://doi.org/10.1038/srep40497
Chen L, Chang BK, Lu Y, Yang W, Tatarchuk BJ (2002) Selective catalytic oxidation of CO for fuel cell application. Fuel Chem Div Prepr 47:609–610
Korotkikh O, Farrauto R (2000) Selective catalytic oxidation of CO in H2: fuel cell applications. Catal Today. https://doi.org/10.1016/S0920-5861(00)00426-0
Saavedra J, Doan HA, Pursell CJ, Grabow LC, Chandler BD (2014) The critical role of water at the gold-titania interface in catalytic CO oxidation. Science. https://doi.org/10.1126/science.1256018
An K, Alayoglu S, Musselwhite N, Plamthottam S, Melaet G, Lindeman AE, Somorjai GA (2013) Enhanced CO oxidation rates at the interface of mesoporous oxides and Pt nanoparticles. J Am Chem Soc. https://doi.org/10.1021/ja4088743
Ferstl P, Mehl S, Arman MA, Schuler M, Toghan A, Laszlo B, Lykhach Y, Brummel O, Lundgren E, Knudsen J, Hammer L, Schneider MA, Libuda J (2015) Adsorption and activation of CO on Co3O4(111) thin films. J Phys Chem. https://doi.org/10.1021/acs.jpcc.5b04145
Eid K, Ahmad YH, Mohamed AT, Elsafy AG, Al-Qaradawi SY (2018) Versatile synthesis of Pd and Cu co-doped porous carbon nitride nanowires for catalytic CO oxidation reaction. Catalysts. https://doi.org/10.3390/catal8100411
Acknowledgements
AS gratefully acknowledges the support of the Bolyai Janos Research Fellowship of the Hungarian Academy of Science and the “UNKP-21-5-SZTE-586” New National Excellence Program as well as the funding provided by the Indo-Hungarian TÉT project (2019-2.1.13-TÉT_IN-2020-00015) of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The Ministry of Human Capacities through the EFOP-3.6.1-16-2016-00014 project and the 20391-3/2018/FEKUSTRAT are acknowledged. ZK is grateful for K_21 138714 and SNN_135918 project for the Hungarian National Research, Development and Innovation Office.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boldizsár, T., Mucsi, R., Szamosvölgyi, Á. et al. Optimalization of ceramic-based noble metal-free catalysts for CO oxidation reactions. Reac Kinet Mech Cat 135, 575–587 (2022). https://doi.org/10.1007/s11144-022-02166-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02166-1