Abstract
This article presents new kinetic studies of the disproportionation of I(+ 3) and of its oxidation by H2O2. It also provides an update of the previously proposed model for reactions of iodine compounds with oxidation numbers from − 1 to + 5 with each other and with H2O2. This model explains the kinetics of several reactions, including the oxidation of iodine by H2O2. We show that the reduction of HOI by H2O2 results from \({\text{HOI }} + {\text{ H}}_{{2}} {\text{O}}_{{2}} \to {\text{HOOI }} + {\text{ H}}_{{2}} {\text{O}}\) followed by the reversible reaction \({\text{HOOI}} \rightleftharpoons {\text{I}}^{ - } + {\text{ H}}^{ + } + {\text{ O}}_{{2}}\). An analysis of previous measurements of the kinetic constant k(HOI + H2O2) explains the large differences between the values proposed in the literature and gives k(HOI + H2O2) = 6 M−1 s−1. The reversibility of the reaction \({\text{HOOI}} \rightleftharpoons {\text{I}}^{ - } + {\text{ H}}^{ + } + {\text{ O}}_{{2}}\) suggests a new explanation for the effect of oxygen on the Bray–Liebhafsky reaction. H2O2 would oxidize HOOI by a radical mechanism.

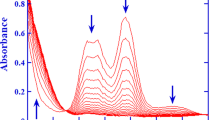
I(+3) autocatalytic disproportionation





Similar content being viewed by others
References
Comprehensive Chemical Kinetics 2004, Volume 40, Chapter 14, Instability, periodic reactions, and chaos.
Schuster HG, Just W (2005) Deterministic chaos—an introduction, 4th edn. Wiley, Weinheim
Čupić ŽD, Taylor AF, Horváth D, Orlik M, Epstein IR (2020) Advances in oscillating reactions. Front Chem 9:292
Ivanović-Šasić AZ, Marković VM, Anić SR, Lj Z, Kolar-Anić and Ž. D. Čupić, (2011) Structures of chaos in open reaction systems. Phys Chem Chem Phys 13:20162–20171
Bosland L, Cantrel L, Girault N, Clement B (2010) Modeling of iodine Radiochemistry in the ASTEC severe accident code: description and application to FPT-2 Phebus test. Nuclear Technol 171:88–107
Fortina C, Fèvre-Nolleta V, Cousin F, Lebègue P, Louisa F (2019) Box modelling of gas-phase atmospheric iodine chemical reactivity in case of a nuclear accident. Atmos Environ 214:116838
Saha S, Roy S, Mathi P, Mondal JA (2020) Adsorption of iodine species (I3−, I−, and IO3−) at the nuclear paint monolayer−water interface and its relevance to a nuclear accident scenario. J Phys Chem A 124:6726–6734
Mahajan AS, Shaw M, Oetjen H, Hornsby KE, Carpenter LJ, Kaleschke L, Tian-Kunze X, Lee JD, Moller SJ, Edwards P, Commane R, Ingham T, Heard DE, Plane JMC (2010) Evidence of reactive iodine chemistry in the Arctic boundary layer. J Geophys Res 115:D20303
Badia A, Reeves CE, Baker AR, Saiz-Lopez A, Volkamer R, Koenig TK, Apel EC, Hornbrook RS, Carpenter LJ, Andrews SJ, Sherwen T, von Glasow R (2019) Importance of reactive halogens in the tropical marine atmosphere: a regional modelling study using WRF-Chem. Atmos Chem Phys 19:3161–3189
Inamdar S, Tinel L, Chance R, Carpenter LJ, Sabu P, Chacko R, Tripathy SC, Kerkar AU, Sinha AK, Venkateswaran Bhaskar P, Sarkar A, Roy R, Sherwen T, Cuevas C, Saiz-Lopez A, Ram K, Mahajan AS (2020) Estimation of reactive inorganic iodine fluxes in the Indian and Southern Ocean marine boundary layer. Atmos Chem Phys 20:12093–12114
Schmitz G, Furrow SD (2018) Kinetics of reactions of iodine inorganic compounds in acidic solutions. In: Physical Chemistry 2018, Proceedings of the 14th Int. Conf. Fundam. Appl. Aspects Phys. Chem. Belgrade, Sept 24–28, Society of Physical Chemists of Serbia pp 271–278
Schmitz G (2010) Iodine oxidation by hydrogen peroxide in acidic solutions, Bray–Liebhafsky reaction and other related reactions. Phys Chem Chem Phys 12:6605–6615
Sharma KR, Noyes RM (1976) Oscillations in chemical systems. 13. A detailed molecular mechanism for the Bray–Liebhafsky reaction of iodate and hydrogen peroxide. J Am Chem Soc 98(15):4345–4361
Schmitz G (1999) Effects of oxygen on the Bray–Liebhafsky reaction. Phys Chem Chem Phys 1:4605
Ševčik P, Kissimonová K, Adamčikova L (2000) Oxygen production in the oscillatory Bray–Liebhafsky reaction. J Phys Chem A 104:3958–3963
Ševčik P, Adamčikova L (1998) Effect of a gas bubbling and stirring on the oscillating Bray–Liebhafsky reaction. J Phys Chem A 102:1288–1291
Ball JM, Hnatiw JB (2001) The reduction of l2 by H2O2 in aqueous solution. Can J Chem 79:304–311
Schmitz G (2008) Buffers catalysis of the iodine(+1) reduction by hydrogen peroxide In Physical Chemistry 2008, Proceedings of the 9th Int. Conf. Fundam. Appl. Aspects Phys. Chem. Society of Physical Chemists of Serbia, Belgrade pp. 219–224
Schmitz G (2009) Iodine(+1) reduction by hydrogen peroxide, Russian. J Phys Chem 83(9):1447
Schmitz G, Furrow SD (2012) Kinetics of the iodate reduction by hydrogen peroxide and relation with the Briggs–Rauscher and Bray–Liebhafsky oscillating reactions. Phys Chem Chem Phys 14:5711–5717
Schmitz G, Furrow SD (2014) Iodine Inorganic Reactions in Acidic Solutions and Oscillating Reactions, Physical Chemistry 2014, Proceedings of the 12th Int. Conf. Fundam. Appl. Aspects Phys. Chem. Society of Physical Chemists of Serbia, Belgrade pp 320–326
Schmitz G, Furrow SD (2016) Bray–Liebhafsky and non-catalyzed Briggs–Rauscher oscillating reactions. Russ J Phys Chem A 90(2):271–275
Szabo E, Ševčík P (2013) Reexamination of gas production in the Bray−Liebhafsky reaction: what happened to O2 pulses? J Phys Chem A 117:10604–10614
Schmitz G, Lente G (2020) Fundamental concepts in chemical kinetics. ChemTexts 6(1):1
Schmitz G (2004) Inorganic reactions of iodine(+1) in acidic solutions. Int J Chem Kinet 36:480
Schmitz G, Furrow SD (2013) Kinetics of iodous acid disproportionation. Int J Chem Kinet 48(8):525–530
Schmitz G, Nullclines (2021) A Simple Explanation of Complicated Phenomena, In Physical Chemistry 2021, Proceedings of the 15th Int. Conf. Fundam. Appl. Aspects Phys. Chem., pp. 222–229, Belgrade, Sept 20–24, Society of Physical Chemists of Serbia, 2021. ISBN 978-86-82475-40-8.
Schmitz G, Noszticzius Z, Hollo G, Wittmann M, Furrow SD (2018) Reactions of iodate with iodine in concentrated sulfuric acid. Formation of I(+3) and I(+1) compounds. Chem Phys Lett 691:44
Furrow SD, Cervellati R, Amadori G (2002) New substrates for the oscillating Briggs–Rauscher reaction. J Phys Chem A 106:5841–5850
Schmitz G (2008) Inorganic reactions of iodine(III) in acidic solutions and free energy of iodous acid formation. Int J Chem Kinet 40:647–652
Schmitz G (2011) Iodine oxidation by hydrogen peroxide and Bray–Liebhafsky oscillating reaction: effect of the temperature. Phys Chem Chem Phys 13:7102–7111
Olexová A, Mrákavová M, Melicherčík M, Treindl L (2006) The autocatalytic oxidation of iodine with hydrogen peroxide in relation to the Bray–Liebhafsky oscillatory reaction. Collect Czech Chem Commun 71:91–106
Olexová A, Mrákavová M, Melicherčík M, Treindl L (2010) Oscillatory system I−, H2O2, HClO4: the modified form of the Bray–Liebhafsky reaction. J Phys Chem A 114:7026–7029
Stanisavljev DR, Stevanović KZ, Bubanja IM (2018) Outsized stochasticity of iodine oxidation with hydrogen peroxide and its implications on the reaction mechanism. Chem Phys Lett 706:120–126
Stevanović KZ, Bubanja IM, Stanisavljev DR (2019) Is iodine oxidation with hydrogen peroxide coupled with nucleation processes? J Phys Chem C 123:16671–16680
Jortner J, Ottolenghi M, Stein G (1962) The effect of oxygen on the photochemistry of the iodide ion in aqueous solutions. J Phys Chem 66:2042–2045
Nardello V, Briviba K, Sies H, Aubry J-M (1998) Identification of the precursor of singlet oxygen (1O2, 1∆g) involved in the disproportionation of hydrogen peroxide catalyzed by calcium hydroxide. Chem Commun 5:599–600
Furrow SD (1987) Reactions of iodine intermediates in iodate-hydrogen peroxide oscillators. J Phys Chem 91:2129–2135
Liebhafsky HA (1932) The catalytic decomposition of hydrogen peroxide by the iodine-iodide couple. III. The rate of oxidation, in acid solution, of hydrogen peroxide by iodine. J Am Chem Soc 54:3499–3508
Shin J, Lee Y, von Gunten U (2020) Kinetics of the reaction between hydrogen peroxide and aqueous iodine: implications for technical and natural aquatic systems. Water Res 179:115852
Schmitz G (1909) Kinetics and mechanism of the iodate-iodide reaction and other related reactions. Phys Chem Chem Phys 1999:1
Furuichi R, Liebhafsky HA (1975) Bull Chem Soc Jpn 48:745
Schmitz G (2000) Kinetics of the Dushman reaction at low I- concentrations. Phys Chem Chem Phys 2:4041
Liebhafsky HA, Mohammad A (1933) The kinetics of the reduction in acidic solution of hydrogen peroxide by iodide ion. J Am Chem Soc 55:3977–3986
Lozar J, Lafage B (1994) Kinetics of the oxidation of iodide ions by oxygenated water using spectrophotometry with a microcomputer. Bulletin de l’Union des Physiciens 88(764):895–902
Stanisavljev DR, Milenković MC, Mojović MD, Popović-Bijelić AD (2011) Oxygen centered radicals in iodine chemical oscillators. J Phys Chem A 115:7955–7958
Stanisavljev DR, Milenković MC, Popović-Bijelić AD, Mojović MD (2013) Radicals in the Bray−Liebhafsky oscillatory reaction. J Phys Chem A 117:3292–3295
Kéki S, Székely G, Beck MT (2003) The effect of light on the Bray–Liebhafsky reaction. J Phys Chem A 107:73–75
Stanbury DM (2018) Comment on the principle of detailed balancing in complex mechanisms and its application to iodate reactions. J Phys Chem A 122:3956–3957
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmitz, G.E., Furrow, S.D. Kinetics and mechanism of I(+ 3) reactions and consequences for other iodine reactions. Reac Kinet Mech Cat 135, 1171–1186 (2022). https://doi.org/10.1007/s11144-022-02155-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02155-4