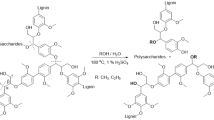
Abstract
Hydrolytic cleavage of C–O bond was systematically studied in acidic/alkaline aqueous solution. For phenethoxybenzene (PEB) hydrolysis, 2-phenylethanol and phenol were formed as main products in AlCl3 solutions, whereas styrene was preferred with Na2CO3. After a quantitative evaluation of the kinetics of elementary steps, it was concluded that both AlCl3 and Na2CO3 promoted protons assisted C–O bond scission in H2O, whereas alkaline enhanced the dehydration of produced 2-phenylethanol. Kinetic and isotope labeling experiments demonstrated that the hydrolytic rates of PEB were first order with respect to substrate concentrations, hydrolytic cleavage of C–O bond was rate-determining step. The study shed light on the promotional effects and hydrolytic mechanism over acid/base-catalyzed lignin depolymerization in water.
Graphic abstract






Similar content being viewed by others
References
Perego C, Pollesel P (2010) Advances in aromatics processing using zeolite catalysts. Adv Nanoporous Mater 1:97
Kim SJ, Han GF, Jung SM, Jeon JP, Shin SH, Kim SW, Jeon IY, Baek JB (2019) Oxidative dehydrogenation of ethylbenzene into styrene by Fe-graphitic catalysts. ACS Nano 13:5893
Suástegui M, Shao Z (2016) Yeast factories for the production of aromatic compounds: from building blocks to plant secondary metabolites. J Ind Microbiol Biot 43:1611
Hua D, Xu P (2011) Recent advances in biotechnological production of 2-phenylethanol. Biotechnol Adv 29:654
Kirm I, Medina F, Rodríguez X, Cesteros Y, Salagre P, Sueiras JE (2005) Preparation of 2-phenylethanol by catalytic selective hydrogenation of styrene oxide using palladium catalysts. J Mol Catal A 239:215
Neumann GT, Pimentel BR, Rensel DJ, Hicks JC (2014) Correlating lignin structure to aromatic products in the catalytic fast pyrolysis of lignin model compounds containing β-O-4 linkages. Catal Sci Technol 4:3953
Gierer J (1980) Chemical aspects of kraft pulping. Wood Sci Technol 14:241
Zhao C, He J, Lemonidou AA, Li X, Lercher JA (2011) Aqueous-phase hydrodeoxygenation of bio-derived phenols to cycloalkanes. J Catal 280:8
Roberts V, Fendt S, Lemonidou AA, Li X, Lercher JA (2010) Influence of alkali carbonates on benzyl phenyl ether cleavage pathways in superheated water. Appl Catal B 95:71
Deuss PJ, Scott M, Tran F, Westwood NJ, de Vries JG, Barta K (2015) Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J Am Chem Soc 137:7456
Konnerth H, Zhang J, Ma D, Prechtl MHG, Yan N (2015) Base promoted hydrogenolysis of lignin model compounds and organosolv lignin over metal catalysts in water. Chem Eng Sci 123:155
Zhu G, Shi S, Zhao L, Liu M, Gao J, Xu J (2020) Catalytic activation of carbon-hydrogen bonds in lignin linkages over strong-base-modified covalent triazine frameworks for lignin oxidative cleavage. ACS Catal 10:7526
Renders T, Schutyser W, Van den Bosch S, Koelewijn SF, Vangeel T, Courtin CM, Sels BF (2016) Influence of acidic (H3PO4) and alkaline (NaOH) additives on the catalytic reductive fractionation of lignocellulose. ACS Catal 6:2055
Li H, Song G (2019) Ru-catalyzed hydrogenolysis of lignin: base-dependent tunability of monomeric phenols and mechanistic study. ACS Catal 9:4054
Jia S, Cox BJ, Guo X, Zhang ZC, Ekerdt JG (2010) Cleaving the β-O-4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-methylimidazolium chloride: an optional strategy for the degradation of lignin. Chemsuschem 3:1078
Yokoyama T (2014) Revisiting the bechanism of β-O-4 bond cleavage during acidolysis of lignin. Part 6: a review. J Wood Chem Technol 35:27
Sarkanen KV, Hoo LH (1981) Kinetics of hydrolysis of erythro-guaiacylglycerol β-(2-methoxyphenyl) ether and its veratryl analogue using HCl and aluminum chloride as catalysts. J Wood Chem Technol 1:11
Sturgeon MR, Kim S, Lawrence K, Paton RS, Chmely SC, Nimlos M, Foust TD, Beckham GT (2014) A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: implications for lignin depolymerization in acidic environments. ACS Sustain Chem Eng 2:472
Schütz MK, Schlegel ML, Libert M, Bildstein O (2015) Impact of iron-reducing bacteria on the corrosion rate of carbon steel under simulated geological disposal conditions. Environ Sci Technol 49:7483
Hashiguchi BG, Young KJH, Yousufuddin M, Goddard WA III, Periana RA (2010) Acceleration of nucleophilic CH activation by strongly basic solvents. J Am Chem Soc 132:12542
Kamireddy SR, Li J, Tucker M, Degenstein J, Ji Y (2013) Effects and mechanism of metal chloride salts on pretreatment and enzymatic digestibility of corn stover. Ind Eng Chem Res 52:1775
Lente G (2018) Facts and alternative facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng 21:76
Lente G (2015) Deterministic kinetics in chemistry and systems biology the dynamics of complex reaction networks. Spring, Berlin
Luo N, Wang M, Li H, Zhang J, Hou T, Chen H, Zhang X, Lu J, Wang F (2017) Visible-light-driven self-hydrogen transfer hydrogenolysis of lignin models and extracts into phenolic products. ACS Catal 7:4571
Wu X, Xie S, Liu C, Zhou C, Lin J, Kang J, Zhang Q, Wang Z, Wang Y (2019) Ligand-controlled photocatalysis of CdS quantum dots for lignin valorization under visible light. ACS Catal 9:8443
Hilgers R, van Dam A, Zuilhof H, Vincken JP, Kabel MA (2020) Controlling the competition: boosting laccase/HBT-catalyzed cleavage of a β-O-4′ linked lignin model. ACS Catal 10:8650
Jarvis MW, Daily JW, Carstensen HH, Dean AM, Sharma S, Dayton DC, Robichaud DJ, Nimolos MR (2011) Direct detection of products from the pyrolysis of 2-phenethyl phenyl ether. J Phys Chem A 115:428
Westermark U, Samuelsson B, Lundquist K (1995) Homolytic cleavage of the β-ether bond in phenolic β-O-4 structures in wood lignin and in guaiacylglycerol-β-guaiacyl ether. Res Chem Intermed 21:343
Meng Q, Yan J, Liu H, Chen C, Li S, Shen X, Song J, Zheng L, Han B (2019) Self-supported hydrogenolysis of aromatic ethers to arenes. Sci Adv 5:6839
Wu X, Fu J, Lu X (2013) Kinetics and mechanism of hydrothermal decomposition of lignin model compounds. Ind Eng Chem Res 52:5016
Yan J, Meng Q, Shen X, Chen B, Sun Y, Xiang J, Liu H, Han B (2020) Selective valorization of lignin to phenol by direct transformation of Csp2-Csp3 and C-O bonds. Sci Adv 6:1951
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552
Acknowledgements
The research is supported financially by NSFC of China (22002113, 21878237, 22072057, 21703179) and the project from Wuhan Institute of Technology (K201913). The authors gratefully acknowledge funding through Science and Technology Department of Hubei Province (2020CFB198) and Wuhan Application Foundation and Frontier Project (2018010401011291).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, W., Song, M., Jiang, X. et al. Hydrolytic cleavage of lignin derived C-O bonds by acid/base catalysis in water. Reac Kinet Mech Cat 133, 371–382 (2021). https://doi.org/10.1007/s11144-021-01990-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-01990-1