Abstract
Ethanol steam reforming was studied over Ni supported catalysts. The effects of support (Al2O3, Al2O3–ZnO, and Al2O3–CeO2), metal loading, catalyst activation method, and steam-to-ethanol molar feed ratio were investigated. The properties of catalysts were studied by N2 physisorption, TPD-CO2, X-ray diffraction, and temperature programmed reduction. After activity tests, the catalysts were analyzed by TOC analysis. The catalytic activity measurements showed that the addition either of ZnO SSor CeO2 to alumina enhances both ethanol conversion and promotes selectivity towards hydrogen formation. The same effects were observed for catalysts with higher metal loadings. High process temperature and high water-to-ethanol ratio were found to be beneficial for hydrogen production. An extended catalyst stability tests showed no loss of activity over 50 h on reaction stream. The TOC analysis of spent catalysts revealed only insignificant amounts of carbon deposit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fuel cell and hydrogen technologies are recognized as one of the most promising solutions that address both energy security and environmental concerns, in addition to being more energy-efficient than diesel or internal combustion engines [1]. The conventional methods of production of hydrogen are based on steam reforming of natural gas, partial oxidation of methane, and coal gasification [2]. Among the different feedstocks available, alcohols are very promising candidates as they can be easily reformed into hydrogen-rich mixture at relatively mild conditions. Particularly, the ethanol is of great interest because of ease of storage and transportation as well as low toxicity and volatility compared to methanol, that have been the first alcohol used to generate hydrogen. Moreover, bioethanol is a renewable source of energy and can be easily produced in large quantities via fermentation of sugar containing raw materials from agriculture. More importantly, use ethanol in fuel cell has the significant advantage of being nearly CO2 neutral as the amount of carbon dioxide produced by steam reforming is consumed for biomass growth, offering a nearly closed carbon loop [3].
The ethanol reforming process involves the following main reactions: steam reforming (SRE, Eq. 1) followed by water–gas shift (WGS, Eq. 2) and selective oxidation of CO (Eq. 3).
The process is usually carried out in the temperature range from 200 to 650 °C under atmospheric pressure. Currently, the metals that are selected for this reaction can be classified into two groups: noble metals including Rh, Pt, Pd, and Ru, and non-noble metals that are mainly based on Ni and Co [4]. Usually, these catalysts are dispersed in a matrix formed by pure or mixed oxides like Al2O3, SiO2, ZrO2, MgO and others [4]. Although many of these catalyst exhibit high activity in the reforming process, they can easily undergo deactivation due to formation and subsequent accumulation of carbon deposit [4]. It occurs due to various side reactions such as Boudouard reaction (Eq. 4), decomposition of methane (Eq. 5) formed either by the ethanol decomposition or methanation of carbon oxides (CO/CO2) or decomposition and/or polymerization of ethylene formed by the dehydratation of ethanol under the reforming conditions.
Nickel-based catalysts are particularly sensitive for carbon deposition [5]. On the other hand, noble metal-based catalysts incur less coke formation due to their high redox capacity, however, their high cost and limited availability restricts their widespread application in industry. Therefore, a great deal of effort has been devoted to suppress coke formation reactions when non-noble metal catalyst is applied. In general, the catalysts are modified by addition of second metals, basic additives or redox active promoters [6]. The use of second metal can give rise to different structures like alloys which are more resistant to carbon deposition due to decreased solubility and diffusion of carbon atoms in metal nanoparticles [7, 8]. The promotion effects of basic oxides (MgO, Na2O, CaO) arise from neutralizing the acidity of catalyst support, e.g. alumina, and, by doing so, inhibiting ethanol dehydration reaction [9]. The other oxides, such ZrO2, CexZr1−xO2, ZnO and La2O2, have been also widely used as they favor ethanol dehydrogenation reactions instead of dehydration [9, 10]. Moreover, the addition of Zn was also reported to weaken the interaction between active phase and support that resulted in improved reduction of nickel particles and therefore their improved activity [11]. The promoting effect was also reported in the case of CeO2–Al2O3 catalytic systems. For instance, the addition of CeO2 to catalytic system can favor direct oxidation of coke as cerium oxide possess oxygen storage capacity, oxygen vacancies, and high oxygen mobility [12]. The formation and gasification of carbon deposit during the reforming reactions are also depend significantly on the process conditions, such as temperature, type and concentration of reaction gasses [13]. Despite the great deal of research available in the literature, the choice of the most optimal catalyst composition is still rather difficult due to different experimental conditions.
The main goal of the present research work was to develop the efficient and stable Ni-based catalysts for steam reforming of ethanol as well as to optimize process conditions (temperature, the composition of the gas mixture) for hydrogen production. The efforts have been devoted to investigation of effects of support (Al2O3, Al2O3–ZnO, and Al2O3–CeO2), metal loading, catalyst activation method, and steam-to-ethanol molar feed ratio on the course of ethanol reforming process.
Experimental methodology
Catalytic material preparation
Support material
The Al2O3 was prepared by precipitation method from an aqueous solution of Al(NO3)3·9H2O (1 M, AR grade) and using a concentrated ammonia solution as a precipitating agent. The ammonia solution (2 M) was added at the rate of 10 mL min−1 to the aluminium nitrate solution placed in a thermostat. The precipitation was carried out at 40 °C. The obtained precipitate was filtered off under vacuum, washed with deionized water until the filtrate became neutral, dried at 120 °C for overnight, and calcined at 400 °C for 4 h under air. The Al2O3–CeO2 and Al2O3–ZnO supports were obtained by co-precipitation method from corresponding nitrate salts (AR grade). The synthesis procedure was similar as this described above. The nominal loading of CeO2 and No was 20 wt%.
Preparation of Ni catalysts
The nickel supported catalysts were prepared by traditional wetness impregnation method using aqueous solution of nitrate salt (1 M, AR grade). The estimated quantities of the precursor solution were added dropwise to supports (CeO2–Al2O3, ZnO–Al2O3, Al2O3) and, then, the obtained mixture was left at ambient for overnight. Next, the excess of the solvent was evaporated under a vacuum. Finally, the impregnated samples were dried at 110 °C for 4 h and, afterward, they were calcined at 400 °C for 4 h in an air atmosphere. The nominal nickel loadings were 5 and 20%.
Physicochemical characterization
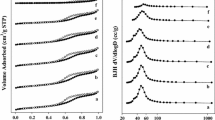
The specific surface area and pore size distribution of the obtained samples were estimated from N2 physical adsorption isotherm at − 195.8 °C, using a Sorptomatic 1900 (Carlo Erba Instruments). The samples were previously outgassed at 300 °C and equilibrated under vacuum for at least 4 h before measuring the adsorption–desorption isotherm. Specific surface areas were evaluated from the measured monolayer capacity (Brunauer–Emmett–Teller method) using the range of relative pressure from ~ 0.05 to 0.33 and the value for nitrogen cross-section 0.162 nm2. The pore size distribution was calculated by the Dollimore and Heal (DH) method from the desorption branch of the isotherm.
Reducibility of the prepared catalysts studied by temperature-programmed reduction measurements were carried out in an automatic AMI-1 instrument (Altamira Instruments, Pittsburgh, PA, USA) in the temperature range of 25–900 °C with a heating rate of 10 °C min−1. The mass of the sample in each test was ~ 0.1 g. The investigated materials were reduced in a mixture of 5% H2–95% Ar with a volumetric flow rate of 40 cm3min−1. Hydrogen consumption was measured using a thermal conductivity detector.
Surface concentrations of basic sites were determined by temperature-programmed desorption of carbon dioxide (CO-TPD). Before TPD experiments, the samples were plugged with helium at 600 °C for 60 min in order to remove any contaminations. After cleaning, the samples were cooled and saturated for 20 min. in flow of pure CO2 at 50 °C. In both cases, the total flow rate was 25 mL min−1. Then, the samples were purged in helium flow until a constant baseline level was attained. TPD measurements were performed in the temperature range 100–600 °C at a rate of 10 °C min−1 using helium as carrier gas. The evolved carbon dioxide were detected by an on-line TCD calibrated by the peak area of known pulses of CO2.
The phase composition of catalysts was investigated by X-ray diffraction analysis. Prior the measurements, the investigated samples were reduced in hydrogen stream (5% H2–95% Ar) at 500 °C for 2 h. The diffraction patterns were collected using a PANalytical X'Pert Pro MPD diffractometer in Bragg Brentano reflection geometry. The diffractometer was equipped with Cu Kα radiation source (λ = 1.5418 Å). Data was collected in the 2θ range of 5°–90° with a step size of 0.0167° and exposure per step of 27 s. Because the raw diffraction data contain some noise, the background during the analysis was subtracted using the Sonneveld, E. J. and Visser algorithm. The data were then smoothed using a cubic polynomial function.
The total organic carbon (TOC) content of the spent samples was determined on a TOC 5000A analyzer (Shimadzu, Japan). Carbon was burnt to CO2 at 900 °C. A nondispersive infrared gas analyzer was used as a detector in all measurements. The instrument was calibrated with the use of pure glucose.
Catalytic activity measurements
The temperature programmed surface reactions were performed in order to select the optimal activation conditions of investigated catalysts. The analysis were performed in the temperature range from 50 to 700 °C with a linear heating rate of 10 °C min−1. Prior to measurements, one sample of Ni/Al2O3 was calcined at 500 °C for 4 h under air while another one was reduced at 500 °C for 4 h in the hydrogen mixture (5% H2–95% Ar). Then, the samples were exposed the reaction mixture. The evolution of the gaseous product was analyzed as a function of temperature using mass spectrometer.
The steam reforming of ethanol was carried out at the atmospheric pressure in a quartz fxed-bed reactor loaded with 0.2 g catalyst. The reaction conditions were as follows: temperature range of 100–600 °C, the ratio of C2H5OH to H2O in the range of 1:3–1:6, and the total fow rate equal 27.5 mL min−1. Before each run, the prepared catalysts were reduced “in situ” in a flow of 5%H2–95%Ar mixture for 1 h at 500 °C. The steady-state activity measurements at each temperature were taken after at least 2 h on stream. The analysis of the reaction organic products was carried out by using an on-line gas chromatograph equipped with an FID and a 10% Carbowax 1500 on a Graphpac column. The CO, CH4, and CO2 concentrations were monitored by a mass spectrometer detector and GC chromatograph equipped with a TCD (150 °C, 60 mA) and a Carbosphere 60/80 (50 °C) column. The hydrogen concentration was measured by a GC chromate graph equipped with a TCD (120 °C, 60 mA) and a Molecular Sieve 5a (120 °C) column. The conversion of methanol and concentration of products were calculated according to the following equations (Eqs. 6 and 7):
Here \(f_{{C_{2} H_{5} OH}}^{in}\) and \(f_{{C_{2} H_{5} OH}}^{out}\) are the inlet and outlet molar flow of ethanol.
Here \(f_{product}^{out}\) is the molar fow rate of the products at the outlet of the reactor.
Results and discussion
The results of specific surface measurements (Table 1) showed that among all investigated samples Al2O3 has the highest surface area of about 100 m2 g−1. The addition of the second metal oxide to aluminum oxide led to decrease of SSA to 76 and 67 m2 g−1 for CeO2 and ZnO, respectively. The same result was observed after the deposition of nickel oxide particles. Irrespective of the support, the specific surface area of catalysts was found to decrease. The lowest specific surface area was observed for the catalyst systems containing the highest amount of nickel oxide (20 wt%). Such result can be due blockage of support pores by the deposited metal particles.
The acidic and basic properties of catalyst support strongly affect the course of reforming reactions. For instance, the basic sites were reported to favor ethanol dehydrogenation step in acetaldehyde formation [14]. The TPD-CO2 measurements for investigated catalysts showed that either the addition of ZnO or increase in nickel metal loading led to decrease in basicity of catalyst surface (Table 2). The total basicity of Al2O3 and Al2O3–ZnO-supported catalysts varied insignificantly and was in the range from 56 to 81 CO2 µmol/gcat. The addition of CeO2, on the other hand, significantly increased the surface basicity of nickel catalyst (5 wt%) up to 146 µmol gcat−1 mainly due to increased amount of weak basic sites. The similar results were reported by Tang et al. [15]. They demonstrated that the presence of ceria led to an increase in either weak or strong sites depending on the concentration of ceria. More particularly, low concentration of ceria gave rise to the strong basic sites while its high concentration resulted in formation of weak ones [15].
The temperature-programmed reduction studies were performed in order to evaluate the reducibility of supported Ni catalysts (Fig. 1). The TPR–H2 of 5%Ni/Al2O3 profile showed a broad reduction peak with the maximum at 530 °C which corresponds to the reduction of Ni species strongly interacted with support [16, 17]. No other prominent peaks for this catalyst were observed. The addition of Ce to 5%Ni/Al2O3 gave a broad reduction peak around 250 °C. Such effect can be assigned to decrease in contact area between NiO and support due to the redistribution of species and changes in support size [18]. The similar low-temperature reduction was observed in the case of 5%Ni/Al2O3–ZnO. Additionally, this catalyst demonstrated high-temperature reduction peak at around 650 °C due to the reduction of ZnO species [19, 20]. In the cases of catalysts with higher metal loading of 20 wt%, the additional hydrogen consumption peak with the maximum at 400–450 °C was observed. This can be assigned to the reduction of NiO species interacting weakly with the support [21]. Similarly, as in the case of 5% nickel catalysts, the addition of either ZnO and CeO2 resulted in the appearance of low-temperature reduction peak. The ease of reducibility of investigated samples can be represented as follows Ni/Al2O3–20%CeO2 > Ni/Al2O3–20%ZnO > Ni/Al2O3.
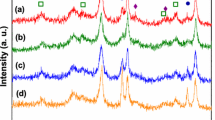
The obtained data is in good agreement with XRD measurements (Fig. 2). The analysis of investigated catalysts was performed after their reduction at 500 °C for 2 h. According to the obtained data, some amount of NiO remained unreduced on the surface of Al2O3 support while in the case of Ni/Al2O3–20%CeO2 catalyst only metallic phase was present.
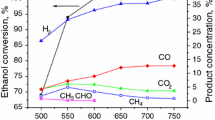
The results of TPSR measurements (Fig. 3) showed that the reforming reaction proceeds more rapidly over the reduced Ni catalyst as indicated by the prominent evolution of hydrogen and carbon monooxide at a temperature above 250 °C. Apart from it, the release of carbon dioxide was also observed at temperatures above 400 °C. The formation of CO2 was due to water gas shift reaction (WGS). Also, it is worth mentioning that a further increase in the temperature led to increase in CO concentration and decrease in CO2 content which can be explained by the change of WGS equilibrium. On the contrary, in the case of calcined catalyst, the reaction started only at temperature around 350–400 °C as evidenced by gradual increase of hydrogen concentration. The effect was also accompanied by simultaneous formation of both carbon mono and dioxide. Similarly, as in the case of reduced sample, the high temperatures favor the formation of hydrogen and carbon monooxide.
In the next step, the investigation of influence of reduction temperature, metal loading and reaction mixture composition (steam to ethanol ratio) was performed (Fig. 4). According to the obtained results, the Ni catalyst reduced at higher temperature was more active than that reduced at 300 °C. Such results imply that the activity of catalyst in the investigated reaction is government significantly by the completeness of reduction of nickel oxide to metallic form. Also, as can be seen, the higher steam to ethanol ratio has pronounce effect on both the conversion of ethanol and selectivity towards hydrogen formation. The highest activity in terms of conversion and selectivity was achieved at temperature 350 °C and ethanol to steam ratio equal to 1:6. The pronounce effect of increase in water vapor concentration can be explained by shifting the balance of water gas shift reaction towards the formation of hydrogen and carbon dioxide.
The results of activity measurements also showed that the increase in nickel loading is beneficial for the activity of investigated samples irrespectively of the catalyst support used. For instance, in the case of Ni/Al2O3 catalyst, the sample with Ni concentration of 20 wt% exhibited almost 90% conversion at temperature as low as 300 °C while sample with 5% of Ni was completely inactive at temperatures up to 350 °C (Fig. 5). It also worth mentioning that similar as in TPSR measurements, the higher reaction temperature favors the formation of hydrogen. As discussed earlier such effect is due to reverse water gas shifting reaction. Moreover, the decomposition/reforming of organic side products (i.e. methanol and dimethyl ether) observed at high temperature also enhances the formation of hydrogen (Table 2).
The same results were obtained for other catalytic systems. It was shown that addition to the alumina support either ZnO or CeO2 enhance both the ethanol conversion and hydrogen selectivity of investigated samples (Table 3). The most pronounce effect was observed in the case of 5%Ni/Al2O3–CeO system when the conversion of ethanol increased by 1.5 times in comparison with unmodified catalyst. Similarly, as in the case of Ni/Al2O3, the increase in nickel concentration to 20 wt% led to complete conversion of ethanol over promoted catalytic systems at 350 °C. The highest selectivity towards hydrogen formation was observed over 20% Ni/Al2O3–ZnO at 600 °C.
As it was mentioned earlier, the nickel catalysts are susceptible to deactivation due to their oxidation as well as formation and subsequent accumulation of carbon deposit on surface. Therefore, it was attempted to perform long-term stability tests of selected catalysts (Al2O3 and Ni/Al2O3–CeO2). The reactions were carried out for 50 h under the same operating conditions as those mentioned previously for the short duration runs. The properties of the spent catalysts were investigated using TOC analysis. The results of the long-term test revealed the stable activities of the investigated catalysts over 50 h of operation with essentially no loss in efficiency (Fig. 6). The product distributions did not change significantly with time and were quite the same as those reported previously for short-term runs. The TOC analysis of the spent catalysts did not show any significant amount of carbon deposits on the surface of catalyst (Table 4).
Conclusions
Steam reforming of ethanol was investigated over nickel catalysts on Al2O3 supports that were either unpromoted or promoted with CeO2 and ZnO. The promoted catalysts showed greater activity and a higher hydrogen yield than the unpromoted catalysts. Among all investigated samples, Ni/Al2O3–CeO2, was found to be the most active one. This can be related to both high concentration of weak basic sites and its enhanced reducibility. Moreover, high nickel concentration, high reaction temperature, and high water-to-ethanol molar ratio were found to be beneficial for hydrogen production. Also, no significant amount of carbon deposit was observed on the surface of used catalyst under investigated conditions.
Change history
08 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11144-021-02095-5
Reference
Ellis MW, Von Spakovsky MR, Nelson DJ (2001) Fuel cell systems: efficient, flexible energy conversion for the 21st century. Proc IEEE. https://doi.org/10.1109/5.975914
Nikolaidis P, Poullikkas A (2017) A comparative overview of hydrogen production processes. Renew Sustain Energy Rev 67:597–611
Kugai J, Velu S, Song C (2003) Novel CeO2 supported Ni–Rh bimetallic catalysts for reforming of bio-ethanol to produce hydrogen for fuel cells. Prepr Pap-Am Chem Soc Div Fuel 48:754–755
Contreras JL, Salmones J, Colín-Luna JA et al (2014) Catalysts for H2 production using the ethanol steam reforming (a review). Int J Hydrog Energy 39:18835–18853. https://doi.org/10.1016/j.ijhydene.2014.08.072
Theofanidis SA, Galvita VV, Poelman H et al (2018) Mechanism of carbon deposits removal from supported Ni catalysts. Appl Catal B 239:502–512. https://doi.org/10.1016/j.apcatb.2018.08.042
Li S, Gong J (2014) Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem Soc Rev 43:7245–7256. https://doi.org/10.1039/c4cs00223g
Bernardo CA, Alstrup I, Rostrup-Nielsen JR (1985) Carbon deposition and methane steam reforming on silica-supported NiCu catalysts. J Catal 96:517–534. https://doi.org/10.1016/0021-9517(85)90320-3
Wang H, Blaylock DW, Dam AH et al (2017) Steam methane reforming on a Ni-based bimetallic catalyst: density functional theory and experimental studies of the catalytic consequence of surface alloying of Ni with Ag. Catal Sci Technol 7:1713–1725. https://doi.org/10.1039/c7cy00101k
Masiran N, Vo DVN, Salam MA, Abdullah B (2016) Improvement on coke formation of CaO–Ni/Al2O3 catalysts in ethylene production via dehydration of ethanol. Procedia Eng 148:1289–1294. https://doi.org/10.1016/j.proeng.2016.06.529
Zhang L, Liu J, Li W et al (2009) Ethanol steam reforming over Ni–Cu/Al2O3–MyOz (M = Si, La, Mg, and Zn) catalysts. J Nat Gas Chem 18:55–65. https://doi.org/10.1016/S1003-9953(08)60078-X
Masoom Nataj SM, Alavi SM, Mazloom G (2019) Catalytic performance of Ni supported on ZnO–Al2O3 composites with different Zn content in methane dry reforming. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.5887
Damaskinos M et al (2019) The effect of CeO2 preparation method on the carbon pathways in the dry reforming of methane on Ni/CeO2 studied by transient techniques. Catalysts 9:1–24
Shtyka O, Zakrzewski M, Ciesielski R et al (2020) Efficient removal of the carbon deposits formed during the mixed methane reforming over Ni/Al2O3. Korean J Chem Eng 37:209–215. https://doi.org/10.1007/s11814-019-0419-3
Lin J, Chen L, Choong CKS et al (2015) Molecular catalysis for the steam reforming of ethanol. Sci China Chem. https://doi.org/10.1007/s11426-014-5262-0
Tang L, Yamaguchi D, Wong L et al (2011) The promoting effect of ceria on Li/MgO catalysts for the oxidative coupling of methane. Catal Today 178:172–180. https://doi.org/10.1016/j.cattod.2011.07.014
Yaakob Z, Bshish A, Ebshish A et al (2013) Hydrogen production by steam reforming of ethanol over nickel catalysts supported on sol gel made alumina: influence of calcination temperature on supports. Mater (Basel). https://doi.org/10.3390/ma6062229
Yang L, Pastor-Pérez L, Gu S et al (2018) Highly efficient Ni/CeO2–Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: effect of selected transition metal promoters. Appl Catal B 232:464–471. https://doi.org/10.1016/j.apcatb.2018.03.091
Charisiou ND, Siakavelas GI, Dou B et al (2019) Nickel supported on AlCeO3 as a highly selective and stable catalyst for hydrogen production via the glycerol steam reforming reaction. Catalysts. https://doi.org/10.3390/catal9050411
Xu J, Su X, Liu X et al (2016) Methanol synthesis from CO2 and H2 over Pd/ZnO/Al2O3: catalyst structure dependence of methanol selectivity. Appl Catal A. https://doi.org/10.1016/j.apcata.2016.01.006
Zhang F, Xu X, Qiu Z et al (2020) Improved methanol synthesis performance of Cu/ZnO/Al2O3 catalyst by controlling its precursor structure. Green Energy Environ. https://doi.org/10.1016/j.gee.2020.11.027
Leal E, Neiva LS, Sousa JPLML et al (2010) Evaluation of glycine excess over NiAl2O4 catalysts prepared by combustion reaction for steam methane reforming. Mater Sci Forum 660–661:916–921
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shtyka, O., Dimitrova, Z., Ciesielski, R. et al. Steam reforming of ethanol for hydrogen production: influence of catalyst composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and process conditions. Reac Kinet Mech Cat 132, 907–919 (2021). https://doi.org/10.1007/s11144-021-01945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-01945-6