Abstract
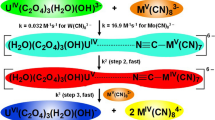
The oxidation of the tetrakisoxalatouranate(IV) ion by the hexacyanoferrate(III) ion was studied in an oxalate buffer medium. The reaction was first order in both UIV(C2O4)44– and Fe(CN)63–, and a second order rate constant of k2av = (5.46 ± 0.04) × 10–2 M−1 s−1 at 34.9 °C was obtained. The reaction is inversely proportional to [H+] and a pKa of 5.99 was found for the deprotonation of UIV(C2O4)3(H2O)22−. Activation parameters have been obtained by application of the Eyring equation. Minimum excess concentrations of UIV(C2O4)44– over Fe(CN)63– for the kinetic study under pseudo-order conditions were evaluated following literature guidelines and also compared to results from a direct second order treatment of data. Contradictory to general beliefs, a fivefold excess of UIV(C2O4)44– was found to be sufficient for a pseudo-order kinetic treatment. The need to obtain results from traditional approximation methods, for example utilizing the linearized Eyring equation to obtain activation enthalpies (∆H#) and entropies (∆S#), was demonstrated not to be required anymore as modern computational capabilities utilizing least square fitting programs allows ∆H# and ∆S# to be obtained directly from temperature and rate constant data utilising the exponential Eyring equation easily and more accurately. A reaction mechanism for the reaction is proposed.
Graphic abstract









Similar content being viewed by others
References
Thyagarajan BS (1958) Oxidations by ferricyanide. Chem Rev 58:439–460
Sharanabasamma K, Tuwar SM (2010) Kinetics and mechanism of oxidation of DL-methionine by hexacyanoferrate(III) in aqueous alkaline medium. J Sulfur Chem 31:177–187
Shimpi R (2019) A review of kinetics of oxidation of organic compounds by hexacyanoferrate(III). RJLBPCS 5:164–181
Majid YA, Howlett KE (1968) Kinetics and mechanisms of redox reactions in aqueous solutions. Part I. The reaction between cyanoferrate (III) and iodide. J Chem Soc A. https://doi.org/10.1039/J19680000679
Agrawal MC, Jindal VK, Mushran SP (1970) Kinetics of arsenite-ferricyanide reaction in an alkaline medium. J Inorg Nucl Chem 32:1257–1262
Jindal VK, Agrawal MC, Mushran SP (1970) Oxidation of hydrazine by alkaline ferricyanide in water-methanol mixtures. Z Naturforsc 25:188–190
Jindal VK, Agrawal MC, Mushran SP (1971) Mechanism of osmium (VIII)-catalysed oxidation of selenium (IV) by aqueous alkaline ferricyanide ion. J Chem Soc A. https://doi.org/10.1039/J19710000622
Jindal VK, Agrawal MC, Mushran SP (1971) Mechanism of osmium (VIII) catalyzed oxidation of tellurium (IV) by alkaline ferricyanide ion. J Inorg Nucl Chem 33:2469–2475
Agrawal MC, Mushran SP (1968) Oxidation of thiourea and thioacetamide by alkaline hexacyanoferrate (III). J Phys Chem 72:1497–1501
Howlett KE, Wedzicha BL (1976) Kinetics of the reaction between hexacyanoferrate (III) and thiosulphate ions. The initial reaction. Inorg Chim Acta 18:133–138
Brown A, Higginson WCE (1967) Oxidation of non-metallic substrates by metal complexes. Chem Commun 15:725–726
Swinehart JH (1967) The kinetics of the hexacyanoferrate (III)-sulphite reaction. J Inorg Nucl Chem 29:2313–2320
Speakman PT, Waters WA (1955) Kinetic features of the oxidation of aldehydes, ketones, and nitroparaffins with alkaline ferricyanide. J Chem Soc. https://doi.org/10.1039/JR9550000040
Yerneni KK, Cholkar K, Guin M, Nayak AN, Ananda S, Gowda NMM (2015) Oxidation of glycylglycine by ferricyanide in acid medium: kinetics and mechanism. Cogent Chem 1:1087296
Newton TW, Baker FB (1967) Aqueous oxidation-reduction reactions of uranium, neptunium, plutonium, and americium. Lanthanide/Actinide Chem 20:268–295
Newton TW (1959) The kinetics of the reaction between Pu (IV) and U (IV). J Phys Chem 63:1493–1497
Harkness AC, Halpern J (1959) Kinetics of the oxidation of uranium(IV) by thallium(III). J Am Chem Soc 81:3526–3529
Betts RH (1955) Kinetics of the oxidation of uranium (IV) by iron (III) in aqueous solutions of perchloric acid. Can J Chem 33:1780–1791
Baker FB, Newton TW (1961) The reaction between uranium(IV) and hydrogen peroxide. J Phys Chem 65:1897–1899
Halpern J, Smith JG (1956) Kinetics of the oxidation of uranium (IV) by molecular oxygen in aqueous perchloric acid solution. Can J Chem 34:1419–1427
Baker FB, Newton TW, Kahn M (1960) The kinetics of the reaction between uranium(IV) and cerium(IV). J Phys Chem 64:109–112
Hassan RM, Mousa MA, El-Shatovry SA (1988) Kinetics of oxidation of uranium(IV) by permanganate ion in aqueous perchlorate media. J Chem Soc Dalton Trans 3:601–603
Hassan RM (2011) A mechanistic approach of the kinetics of oxidation of uranium(IV) by hexachloroplatinate(IV) in aqueous perchorate solutions. Evidence of the formation of a binuclear intermediate complex. J Phys Chem A 115:13338–13345
Hassan RM, Kojima T, Fukotumi H (1980) Kinetic study of the oxidation of uranium(IV) by ferricyanide ions in aqueous solutions. Bull Res Lab Nucl React Jpn 12:41–47
Hassan RM (1991) Kinetics of reaction of uranium(IV) and hexachloroiridate(IV) in acid perchlorate solutions. Evidence for a binuclear intermediate. J Chem Soc Dalton Trans 11:3003–3008
Bolotova GT, Golovnya VA (eds) (1964) Kompleksnye soedineniya urana, Akademiya nauk SSSR, Institut obschei i neorganicheskoi kimii (Complex uranium compounds, Academy of Sciences of the USSR, Institute of General and Inorganic Chemistry), Chapter 19
Kraus KA, Nelson F (1950) Hydrolytic behavior of metal ions. I. The acid constants of uranium(IV) and plutonium(IV). J Am Chem Soc 72:3901–3906
Lente G (2015) Deterministic kinetics in chemistry and systems biology. Springer, Cham, pp 61–65. https://doi.org/10.1007/978-3-319-15482-4
Marchi LE (1950) Potassium tetraoxalatouranate(IV). Inorg Synth 3:169–170
Dennis CR, Fourie E, Margerum DW, Swarts JC (2020) Kinetic advantage of inner sphere electron transfer reactions of copper(III, II) peptide complexes with cyano complexes of iron, molybdenum and tungsten. Transit Met Chem 45:147–157
Dennis CR, Margerum DW, Fourie E, Swarts JC (2020) A kinetic study of the electron-transfer reactions of nickel(III, II) tripeptide complexes with cyano complexes of molybdenum, tungsten and iron. Inorg Chem 59:11695–11703
“Scientist” software, version 2, MicroMath, Saint Louis, Missouri, USA
Wilkins RG (1991) The study of kinetics and mechanism of transition metal complexes, 2nd edn. Allyn and Bacon, Boston, p p8
Beller G, Szabo M, Lente G, Fabian I (2016) Formation of 1,10-phenanthroline-N, N′-dioxide under mild conditions: the kinetics and mechanism of the oxidation of 1,10-phenanthroline by peroxomonosulfate ion (oxone). J Org Chem 81:5345–5353
Zakharova FA, Moskvin AI (1960) The solubility product of uranium (IV) oxalate and the composition and dissociation constants of oxalato-uranium (IV) complexes in aqueous solution. Zhur Neorg Khim 5:1228–1233
Bolotova GT, Golovnya VA (eds) (1964) Kompleksnye soedineniya urana, Akademiya nauk SSSR, Institut obschei i neorganicheskoi kimii (Complex uranium compounds, Academy of Sciences of the USSR, Institute of General and Inorganic Chemistry), Chapter 21
Sharpe AG (1976) The chemistry of cyano complexes of the transition metals. Academic Press, London, p p116
Swarts JC, Aquino MAS, Han J, Lam KY, Sykes AG (1995) Kinetic studies on the reduction of the tyrosyl radical of the R2 subunit of E. coli ribonucleotide reductase. Biochim et Biophys Acta 1247:215–224
Han J, Swarts JC, Sykes AG (1996) Kinetic studies on the hydrazine and phenyl hydrazine reductions of Escherichia coli R2 subunit of ribonucleotide reductase. Inorg Chem 35:4629–4634
Laidler KJ (1963) Reaction kinetics, vol 2. Pergamon Press Ltd, London
Jain DVS, Nandel FS (1967) Role of alkali-metal cations on the kinetics of reaction between hexacyanoferrate (III) and sulphite ions. J C S Dalton 9:949–951
Dennis CR, Basson SS, Leipoldt JG (1983) Kinetics and salt effects of the reduction of octacyanomolybdate(V) and octacyanotungstate(V) by sulphite ions. Polyhedron 2:1357–1362
Domingo PL, García B, Leal JM (1990) Acid behavior of the ferricyanide ion in perchloric acid media. Spectrophotometric and kinetic study. Can J Chem 68:228–235
Lente G, Fabien I, Poe J (2005) A common misconception about the Eyring equation. New J Chem 29:759–760
Lente G (2018) Facts and alternative facts in chemical kinetics: Remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng 21:76–83
Dennis CR, Potgieter IM, Langner EHG, Fourie E, Swarts JC (2019) The oxidation of acetaldehyde by the octacyanomolybdate(V) ion in an aqueous alkaline medium. Transit Met Chem 44:161–165
Acknowledgements
The authors acknowledge the Central Research Fund of the University of the Free State, Bloemfontein, South Africa and the South African National Research Foundation Grant 105725 (EF) for funding.
Author information
Authors and Affiliations
Contributions
GJvZ: Investigation. EF: Investigation, writing, editing. CRD: Investigation, Writing, review, editing, project admin. SSB: Conceptualisation, methodology, review, editing, supervision. JCS: Conceptualization, methodology, review, editing, project admin supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors are not aware of any conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dennis, C.R., van Zyl, G.J., Fourie, E. et al. A kinetic study of the oxidation of the tetrakisoxalatouranate(IV) ion by the hexacyanoferrate(III) ion in an oxalate buffer medium. Reac Kinet Mech Cat 132, 599–615 (2021). https://doi.org/10.1007/s11144-021-01938-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-01938-5