Abstract
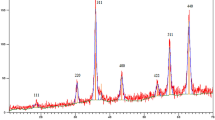
Maghemite nanoparticles modified with polyvinylpyrrolidone were synthesized using the chemical co-precipitation method. Palladium ions have then been immobilized on the PVP/γ-Fe2O3 magnetic support. The Pd-PVP/γ-Fe2O3 catalyst obtained was characterized using X-ray diffraction, thermogravimetry coupled with differential scanning calorimetry and quadrupole mass spectrometry, scanning electron microscopy combined with energy dispersive X-ray analysis, transmission electron microscopy, nitrogen adsorption–desorption and vibrating sample magnetometry. Morphology, microstructure, particle sizes and textural properties of the Pd-PVP/γ-Fe2O3 were compared with those of the unmodified Pd/γ-Fe2O3 catalyst, synthesized using the same method. The polymer has affected the size of γ-Fe2O3 nanoparticles and their agglomeration, forming compact microstructure with decreased mesopores (4.5 nm). Palladium nanoparticles with size of 3–5 nm were found both on the surface of the PVP/γ-Fe2O3 particles (6–10 nm) and inside the pores formed by them. The Pd-PVP/γ-Fe2O3 has demonstrated improved catalytic properties in phenylacetylene hydrogenation under mild conditions of 40 °C and 0.1 MPa, compared to Pd/γ-Fe2O3 and similar catalysts prepared using polyethylene glycol and pectin. The hydrogenation rate of C–C triple bond on Pd-PVP/γ-Fe2O3 achieved 2.8 × 10–6 mol s−1 and selectivity to styrene was 92%. The catalyst showed weak ferromagnetic and soft magnetic properties (MS = 51 emu g−1, Mr = 5.4 emu g−1, and HC = 71 Oe) and, therefore, can be easily recovered with an external magnet and reused for at least 11 runs without significant degradation in the catalytic activity.




Similar content being viewed by others
References
Govan J, Gun’ko YK (2014) Recent advances in the application of magnetic nanoparticles as a support for homogeneous catalysts. Nanomaterials 4:222–241
Shifrina ZB, Bronstein LM (2018) Magnetically recoverable catalysts: beyond magnetic separation. Front Chem 6:298
Abu-Dief AM, Abdel-Fatah SM (2018) Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ J Basic Appl Sci 7:55–67
Shokouhimehr M (2015) Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 5:534–560
Rossi LM, Costa NJS, Silva FP, Wojcieszak R (2014) Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem 16:2906–2933
Chen S-W, Zhang Z-C, Zhai N-N, Zhong C-M, Lee S (2015) The effect of silica-coating on catalyst recyclability in ionic magnetic nanoparticle-supported Grubbs–Hoveyda catalysts for ring-closing metathesis. Tetrahedron 71:648–653
Zhao J, Gui Y, Liu Y, Wang G, Zhang H, Sun Y, Fang S (2017) Highly efficient and magnetically recyclable Pt catalysts for hydrosilylation reactions. Catal Lett 147:1127–1132
Nasir Baig RB, Varma RS (2014) Magnetic carbon-supported palladium nanoparticles: an efficient and sustainable catalyst for hydrogenation reactions. ACS Sustain Chem Eng 2:2155–2158
Kainz QM, Reiser O (2014) Polymer- and dendrimer-coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc Chem Res 47:667–677
Li S, Lieberzeit P, Piletsky S, Turner A (2019) Smart polymer catalysts and tunable catalysis. Elsevier, Amsterdam
Liu G, Wang D, Zhou F, Liu W (2015) Electrostatic self-assembly of Au nanoparticles onto thermosensitive magnetic core–shell microgels for thermally tunable and magnetically recyclable catalysis. Small 11(23):2807–2816
Wang D, Duan H, Lü J, Lü C (2017) Fabrication of thermo-responsive polymer functionalized reduced graphene oxide@Fe3O4@Au magnetic nanocomposites for enhanced catalytic applications. J Mater Chem A 5(10):5088–5097
Pourjavadi A, Tajbakhsh M, Farhang M, Hosseini SH (2015) Copper-loaded polymeric magnetic nanocatalysts as retrievable and robust heterogeneous catalysts for click reactions. New J Chem 39(6):4591–4600
Yang J, Zhu Y, Fan M, Sun X, Wang WD, Dong Z (2019) Ultrafine palladium nanoparticles confined in core–shell magnetic porous organic polymer nanospheres as highly efficient hydrogenation catalyst. J Colloid Interface Sci 554:157–165
Zhang H, Zhu Y, Qiao N, Chen Y, Gao L (2017) Preparation and characterization of carbamazepine cocrystal in polymer solution. Pharmaceutics 9:54
Gill CS, Long W, Jones CW (2009) Magnetic nanoparticle polymer brush catalysts: alternative hybrid organic/inorganic structures to obtain high, local catalyst loadings for use in organic transformations. Catal Lett 131:425–431
Evangelisti C, Nicoletta P, D’Alessio A, Bertinetti L, Botavina M, Vitulli G (2010) New monodispersed palladium nanoparticles stabilized by poly-(N-vinyl-2-pyrrolidone): preparation, structural study and catalytic properties. J Catal 272:246–252
Pandey G, Singh S, Hitkari G (2018) Synthesis and characterization of polyvinyl pyrrolidone (PVP)-coated Fe3O4 nanoparticles by chemical co-precipitation method and removal of Congo red dye by adsorption process. Int Nano Lett 8:111–121
Zulfiqar AS, Khan R, Zeb T, Rahman MU, Burhanullah AS, Khan G, Rahman ZU, Hussain A (2018) Structural, optical, dielectric and magnetic properties of PVP coated magnetite (Fe3O4) nanoparticles. J Mater Sci Mater Electron 29:20040–20050
Zharmagambetova AK, Seitkalieva KS, Talgatov ET, Auezkhanova AS, Dzhardimalieva GI, Pomogailo AD (2016) Polymer-modified supported palladium catalysts for the hydrogenation of acetylene compounds. Kinet Catal 57:360–367
Qiu XY, Meng XS, Mao H, He ZH, Lin YQ, Liu XD, Li DC, Li J (2018) Magnetic nanoparticles prepared by chemically induced transition: structure and magnetization behaviors. Mater Chem Phys 204:328–335
Woo K, Hong J, Choi S, Lee H-W, Ahn J-P, Kim CS, Lee SW (2004) Easy synthesis and magnetic properties of iron oxide nanoparticles. Chem Mater 16:2814–2818
Tranchard P, Duquesne S, Samyn F, Estebe B, Bourbigot S (2017) Kinetic analysis of the thermal decomposition of a carbon fibre-reinforced epoxy resin laminate. J Anal Appl Pyrol 126:14–21
Netto AVG, Santana AM, Mauro AE, Frem RCG, de Almeida ET, Crespi MS, Zorel HE Jr (2005) Thermal decomposition of palladium(II) pyrazolyl complexes; Part II. J Therm Anal Calorim 79:339–342
Arsalani N, Fattahi H, Nazarpoor M (2010) Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym Lett 4:329–338
Mascolo MC, Pei Y, Ring TA (2013) Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 6:5549–5567
Kim YJ, Lee MH, Kim HJ, Lim G, Choi YS, Park NG, Kim K, Lee WI (2009) Formation of highly efficient dye-sensitized solar cells by hierarchical pore generation with nanoporous TiO2 spheres. Adv Mater 21:3668–3673
Yang B, Wei Y, Liu Q, Luo Y, Qiu S, Shi Z (2019) Polyvinylpyrrolidone functionalized magnetic graphene-based composites for highly efficient removal of lead from wastewater. Colloids Surf A 582:123927
Bhatti QA, Baloch MK, Schwarz S, Petzold G (2014) Effect of various parameters on the stability of silica dispersions. J Solut Chem 43:1916–1928
Belykh LB, Skripov NI, Sterenchuk TP, Gvozdovskaya KL, Sanzhieva SB, Schmidt FK (2019) Pd-P nanoparticles as active catalyst for the hydrogenation of acetylenic compounds. J Nanopart Res 21:198
Guettari M, Belaidi A, Abel S, Tajouri T (2017) Polyvinylpyrrolidone behavior in water/ethanol mixed solvents: comparison of modeling predictions with experimental results. J Solut Chem 46(7):1404–1417
Sesigur F, Dasdan DS, Yazici O, Cakar F, Cankurtaran O, Karaman F (2016) Thermodynamical characterization of poly (ethylene glycol) and Tosylate functional-ized poly(ethylene glycol) Interaction with some nonpolar and polar solvents via inverse gas chromatography. Optoelectron Adv Mater Rapid Commun 10(1–2):97–101
Vongsetskul T, Chantarodsakun T, Wongsomboon P, Rangkupan R, Tangboriboonrat P (2015) Effect of solvent and processing parameters on electrospun polyvinylpyrrolidone ultra-fine fibers. Chiang Mai J Sci 42(2):436–442
Sherrington DC (1998) Preparation, structure and morphology of polymer supports. Chem Commun 21:2275–2286
Wu W, Xiao XH, Zhang SF, Peng TC, Zhou J, Ren F, Jiang CZ (2010) Synthesis and magnetic properties of maghemite (γ-Fe2O3) short-nanotubes. Nanoscale Res Lett 5:1474
Talgatov ET, Auyezkhanova AS, Seitkalieva KS, Tumabayev NZ, Akhmetova SN, Zharmagambetova AК (2020) Co-precipitation synthesis of mesoporous maghemite for catalysis application. J Porous Mater 27:919–927
Gutiérrez L, de la Cueva L, Moros M, Mazario E, de Bernardo S, de la Fuente JM, Morales MP, Salas G (2019) Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 30:112001
Acknowledgements
This study was funded by Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan (Grants AP05130377, AP05133114). The authors are grateful to G.I. Dzhardimalieva and A.R. Brodskii for assistance.
Funding
This study was funded by Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan (Grants AP05130377, AP05133114).
Author information
Authors and Affiliations
Contributions
AKZ (Writing—Review & Editing); ETT (Conceptualization, Supervision, Formal analysis, Methodology, Writing- Original draft preparation); ASA (Methodology, Investigation, Data curation); NZT (Investigation, Data curation); FUB (Investigation, Visualization).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zharmagambetova, A.K., Talgatov, E.T., Auyezkhanova, A.S. et al. Effect of polyvinylpyrrolidone on the catalytic properties of Pd/γ-Fe2O3 in phenylacetylene hydrogenation. Reac Kinet Mech Cat 131, 153–166 (2020). https://doi.org/10.1007/s11144-020-01857-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01857-x