Abstract
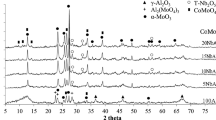
Samples of a commercial hydrotreating catalyst with different initial arsenic content were used in the study light cycle oil (LCO) feed in a hydrotreating unit operating at high pressure (350 °C, 3 MPa) to evaluate the conversion and selectivity of the catalyst in the presence of arsenic. It was determined that arsenic in the catalyst modifies the textural properties and has the strongest influence on the conversion of the HDS reaction. For the first time, evidence is provided that the arsenic replaces cobalt in CoMoS phase. The incorporation of As is proposed to occur in “edge” sites of the molybdenum sulfide disfavoring the C–S cleavage during the HDS reaction, while hydrogenation sites would be favored.
Graphic Abstract





Similar content being viewed by others
References
Eijsbouts S (1999) Life cycle of hydroprocessing catalysts and total catalyst management. Stud Surf Sci Catal 127:21–36. https://doi.org/10.1016/s0167-2991(99)80391-7
Zhang H, Lin H, Zheng Y (2020) Deactivation study of unsupported nano MoS2 catalyst. Carbon Resour Convers 3:60–66. https://doi.org/10.1016/j.crcon.2019.09.003
Tailleur RG (2019) Hydrogenation and hydrodesulfurization in gas phase of light hydrocarbons from hydrocracking, desulfurization and delayed coking. I catalyst deactivation. Chem Eng Sci 210:115195. https://doi.org/10.1016/j.ces.2019.115195
Duarte L, Garzón L, Baldovino-Medrano VG (2019) An analysis of the physicochemical properties of spent catalysts from an industrial hydrotreating unit. Catal Today 338:100–107. https://doi.org/10.1016/j.cattod.2019.05.025
Torres-Mancera P, Ancheyta J, Martínez J (2018) Deactivation of a hydrotreating catalyst in a bench-scale continuous stirred tank reactor at different operating conditions. Fuel 234:326–334. https://doi.org/10.1016/j.fuel.2018.06.122
Rodríguez E, Félix G, Ancheyta J, Trejo F (2018) Modeling of hydrotreating catalyst deactivation for heavy oil hydrocarbons. Fuel 225:118–133. https://doi.org/10.1016/j.fuel.2018.02.085
Zhou J, Zhao J, Zhang J et al (2020) Regeneration of catalysts deactivated by coke deposition: a review. Chin J Catal 41:1048–1061. https://doi.org/10.1016/S1872-2067(20)63552-5
Ferella F (2020) A review on management and recycling of spent selective catalytic reduction catalysts. J Clean Prod 246:118990. https://doi.org/10.1016/j.jclepro.2019.118990
Sarrazin P, Cameron CJ, Barthel Y, Morrison ME (1993) Processes prevent detrimental effects from As and Hg in feedstocks. Oil Gas J 91:86
Opinder Kishan Bhan SOC (2004) United States Patent. US 6759364 B2
Stigter JB, De Haan HPM, Guicherit R et al (2000) Determination of cadmium, zinc, copper, chromium and arsenic in crude oil cargoes. Environ Pollut 107:451–464. https://doi.org/10.1016/S0269-7491(99)00123-2
Maurice V, Ryndin YA, Candy JP et al (2001) Influence of the dispersion of metallic particles on the reaction of triphenylarsine with alumina-supported nickel. J Catal 204:192–199. https://doi.org/10.1006/jcat.2001.3357
Nielsen B, Villadsen J (1984) Poisoning of nickel catalysts by arsenic. Appl Catal 11:123–138. https://doi.org/10.1016/S0166-9834(00)84046-4
Rai A, Escalona G, Betancourt P, Sinha AK (2018) Hydroprocessing of light cycle oil (LCO) over sulfided NiMo supported on hierarchical mesoporous H-ZSM-5 catalyst. React Kinet Mech Catal 125:1099–1112. https://doi.org/10.1007/s11144-018-1423-z
Meng J, Yang J, Fang J et al (2019) Production of liquid fuels from low-temperature coal tar via hydrogenation over CoMo/USY catalysts. React Kinet Mech Catal 127:961–978. https://doi.org/10.1007/s11144-019-01576-y
Yang S, Adjaye J, McCaffrey WC, Nelson AE (2010) Density-functional theory (DFT) study of arsenic poisoning of NiMoS. J Mol Catal A Chem 321:83–91. https://doi.org/10.1016/j.molcata.2010.02.006
Merryfield RN, Gardner LE, Parks GD (1984) Arsenic poisoning of hydrodesulfurization catalysts. Am Chem Soc Div Pet Chem Prepr 29:672–680. https://doi.org/10.1021/bk-1985-0288.ch001
Sie ST (1980) Catalyst deactivation by poisoning and pore plugging in petroleum processing. Elsevier Scientific Publishing Company, Amsterdam
Ryndin YA, Candy JP, Didillon B et al (2001) Surface organometallic chemistry on metals applied to the environment: hydrogenolysis of AsPh3 with nickel supported on alumina. J Catal 198:103–108. https://doi.org/10.1006/jcat.2000.3116
Puig-Molina A, Nielsen LP, Molenbroek AM, Herbst K (2004) In situ EXAFS study on the chemical state of arsenic deposited on a NiMoP/Al2O3 hydrotreating catalyst. Catal Lett 92:29–34. https://doi.org/10.1023/b:catl.0000011082.20411.92
Wagner CD, Davis LE, Zeller MV et al (1981) Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf Interface Anal 3:211–225. https://doi.org/10.1002/sia.740030506
D4294–03 (2003) Standard test method for sulfur in petroleum and petroleum products by energy-dispersive X-ray fluorescence spectrometry. ASTM Int, West Conshohocken, PA
Rana MS, Ramírez J, Gutiérrez-Alejandre A et al (2007) Support effects in CoMo hydrodesulfurization catalysts prepared with EDTA as a chelating agent. J Catal 246:100–108. https://doi.org/10.1016/j.jcat.2006.11.025
Travert A, Dujardin C, Maugé F et al (2006) CO adsorption on CoMo and NiMo sulfide catalysts: a combined IR and DFT study. J Phys Chem B 110:1261–1270. https://doi.org/10.1021/jp0536549
Daage M, Chianelli RR (1994) Structure–function relations in molybdenum sulfide catalysts: the “Rim-Edge” model. J Catal 149:414–427
Acknowledgements
We are grateful to Dr. Omar Ocanto (PDVSA-Intevep) for performing the XPS measurements, and Ch.E. Yraida Díaz for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodriguez, M., Pinto-Castilla, S., Morgado-Vargas, M. et al. Influence of arsenic on light cycle oil hydrodesulfurization over a CoMo catalyst. Reac Kinet Mech Cat 131, 199–211 (2020). https://doi.org/10.1007/s11144-020-01856-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01856-y