Abstract
Reaction rates in a two-step catalytic sequence, when plotted vs adsorption energy of the key or the most abundant surface intermediate, result in volcano shaped curves. In the current work, the optimal catalyst is discussed for structure sensitive reactions, which display dependence of activity on the cluster size of the active catalytic phase. An expression is derived relating the Gibbs energy for formation of the intermediate with the Gibbs energy changes in the overall reaction, difference in adsorption thermodynamics on edges and terraces and the cluster size. The kinetic expressions display dependence of activity vs the Gibbs energy of the adsorbed intermediate formation. Numerical analysis demonstrates that when the overall equilibrium constant K is high and the reaction is thermodynamically very favorable, the maxima in the rates vs the adsorption constant for the optimal catalyst are much broader being less dependent on the cluster size. When structure sensitivity is pronounced, there are smaller differences in the rates for the optimum and less optimal catalysts in comparison with reactions showing weak structure sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selection of an optimum catalyst, i.e. a catalyst, which has a maximum reaction rate, has been of interest for decades starting from the classical works of Sabatier, Balandin and Temkin [1,2,3].
The Sabatier principle providing a conceptual framework for analysis of the optimum catalyst [4], relies on an intuitive concept of an intermediate binding strength of the reactants to the catalyst leading to different types of volcano curves, named after Balandin [2], Tamaru–Tanaka [5] or Sachtler–Fahrenfort [6].
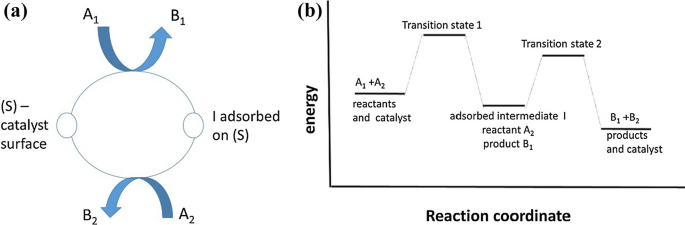
Analysis of the optimal catalyst from a kinetic perspective was performed by Temkin [3] for a two-step sequence (Fig. 1), i.e. a mechanism with two kinetically significant steps [7, 8], and one most abundant surface intermediates
Here A1, A2 are reactants, B1 and B2 are products, S is the surface site and I is an adsorbed intermediate.
In [3], the linear free energy relationship was used, linking kinetics (e.g. rate constant, k) and thermodynamics (equilibrium constant, K) through for example the Brønsted equation:
Here g is a constant and α is the Polanyi parameter (0 < α < 1). This parameter was taken as equal to 0.5 leading to expression of the surface coverage in the case of the optimal catalyst \(\theta = 0.5\). The same result was reported in [9].
A more general treatment in [7] resulted in the following expression for the optimal coverage
Here α1 and α2 are the Polanyi parameters of the first and second steps in the two-step sequence, while \(\omega_{ + 1}^{*}\), etc. are so-called frequencies of steps for the optimal catalyst, e.g. \(\omega_{ + 1}^{*} = k_{ + 1}^{*} P_{{A_{1} }}\). Here \(P_{{A_{1} }}\), etc., correspond to the pressure of the reactant A1. In fact, the treatment below can be used not only to gas-surface heterogeneous catalysis, but also to the liquid/surface catalysis, applying concentrations instead of pressures. The superscript asterisk “*” correspond specifically to the values and parameters (e.g. frequencies, Gibbs energy or equilibrium constants of steps) of the optimum catalyst. Subscripts “ + 1”, “− 1”, “ + 2”, “− 2” denote relevance to the forward and reverse of the first and second steps. In the derivations mentioned above the catalyst surfaces were considered to be uniform, i.e. obeying the Langmuir isotherms without any distinction between surface sites or interactions between adsorbed species. The overall expression for the reaction rate in the two-step sequence on uniform surfaces is [10, 11]:
Analysis of the optimum catalyst, e.g. the catalyst with the highest rate, was done in [3] for the case when the values of Polanyi parameters are equal for both steps being both 0.5. By considering that the rate is at maximum for the lowest value of the denominator in Eq. 4, it was demonstrated already in [3] that for an ideal adsorbed layer the condition of the most active catalyst surface coverage on the optimum catalyst is \(\theta = 0.5\). This result was interpreted as existence of an optimal heat of adsorption for the intermediate I, which corresponds to the coverage equal to 0.5. Later on the treatment was extended to the case when the values of Polanyi parameters are equal for both steps being different from 0.5 [7]. In that instance the condition of the most active catalyst surface coverage on the optimum catalyst is \(\theta = \alpha\). The same result was obtained for the value of the optimum coverage on a surface of a catalyst with adsorbate–adsorbate interactions [12].
For the case of the reaction when the Polanyi parameters for both steps are equal to 0.5 for the optimal catalyst on uniform surfaces it was demonstrated that the Gibbs energy for both steps in mechanism (1) are approximately equal to each other \(\Delta G_{1}^{*} \approx \Delta G_{2}^{*}\) [7]. This result can be interpreted in a way (not explicitly mentioned in [7]) that the Gibbs energy of adsorption of the intermediate for an optimal catalyst is approximately one half of the Gibbs energy of the reaction when \(P_{{A_{2} }} P_{{B_{2} }} \approx P_{{A_{1} }} P_{{B_{1} }}\).
Later development of the theory for the optimum catalyst was associated with an assessment of the optimal adsorption energies through the use of chemical potential [13,14,15], showing for example that the chemical potential of the adsorbed species on an optimal catalyst is approximately a half sum of the chemical potential of the reactants and products in the gas phase, which is analogous to the conclusion following from [7].
The energy span model [16,17,18] was applied [16] to elucidate the adsorption energy for the optimal catalyst using a generic two-step sequence and considering that either the first or the second steps are determining the rate.
An interesting aspect, which was somewhat overlooked in the theoretical treatment mentioned above, is the influence of the catalyst cluster size, even it is well known that catalytic activity in transition metal catalysis can change significantly as a function of the cluster size in the nanometer range, e.g. in the domain between 2 and 20 nm [19,20,21,22,23].
The current study is thus aimed at analysis of the optimum catalyst incorporating the cluster size dependence.
Energetic characteristics of clusters
The approach to explain the dependence of the rate constant on the cluster size was developed in [22] and takes into account differences in the activation energy between edges and terraces, leading to different activities of them in terms of reaction rates and selectivity.
The Gibbs energy of adsorption has the following weighted average form:
Here \(\Delta G_{ads,terraces}\) and \(\Delta G_{ads,edges}\) are respectively Gibbs energies of adsorption on terraces and edges, fractions of these surface sites are \(f_{terraces} ,f_{edges}\).
The relationship between the adsorption constant and the Gibbs energy of adsorption is thus:
Here parameter \(\lambda\) is defined as follows:
\(K_{ads,t}\) corresponds to adsorption on terraces.
In [13], it was demonstrated, that the fraction of edges can be described in a simplified way as \(f_{edges} \approx 1/d_{cluster}\) when \(d_{cluster}\) is the cluster diameter given in nm. Subsequently, the cluster size dependence of the equilibrium constant for adsorption may be written as:
Taking into account the linear free energy (or Brønsted–Evans–Polanyi) relationship between reaction constants k and equilibrium constants K in a series of analogous elementary reactions (Eq. 2) the rate constants can be expressed for different steps in the reaction mechanism (Eq. 1 and Fig. 1) through the respective equilibrium constants. For the first step in the two-step sequence in the forward direction, which can be viewed as adsorption the rate constant, is thus:
For the backward reaction it holds [7]
The equilibrium constant of the first step is then
Analogously to Eq. 6, it can be written for desorption taking into account that \(\Delta G_{des} = - \Delta G_{ads}\)
For the second step in the two-step mechanism it thus holds:
This is in line with the independence of the overall equilibrium constant K = K1K2 on the cluster size and that this constant is a multiplication of the equilibrium constants for the first step and second steps on terraces giving thus \(K = K_{1.t} K_{2.t}\). Subsequently:
Taking Eqs. 10–11 and 15–16 into account, an expression for the two-step sequence (Fig. 1) including the cluster size dependence is:
The optimum rate
Equation 17 can be rewritten as follows:
Or, alternatively:
The optimum catalyst for the two step sequence (Fig. 1) is the one displaying maximum for the forward reaction [7] or minimum for its reciprocal 1/r+. Such analysis was performed in [7] for the two step sequence where the optimum in the rate of the forward reaction was determined for the parameter z = lnK1 or in another form \(z = - \Delta G_{1} /RT\). It was demonstrated [7] that when \(d(1/r_{ + } )/dz = 0\) the following is valid:
After the introduction of expressions for the frequencies of steps containing cluster size dependence one gets:
In [3] and [7], a special case was considered when the Polanyi parameters of both steps are equal to each other \(\alpha_{1} = \alpha_{2}\). Such assumption for the current case of structure sensitivity analysis replacing also \(K_{2.t}\) through K results in:
After some manipulations, an expression for the Gibb energy of the first step in the case of the optimal catalyst is obtained:
Previously, a similar expression without the cluster size dependence was derived in [7] for \({{K}}_{1}^{*}\). When the Polanyi parameters of both steps are equal to each other from (20) one gets:
From the surface coverage of the intermediate in the case of the two step sequence [10, 11]
It follows that for the optimal catalyst \(\theta_{{}}^{*} = \alpha\) independent on the cluster size.
When the Polanyi parameters for two steps are not equal to each other but both steps are irreversible one gets from Eq. 21:
And thus:
For this case the rate for the optimal catalyst is:
After rearrangements Eq. 28 takes the following form:
This can be further modified to:
Here \(p_{1} = g_{1} P_{{A_{1} }}\) and \(p_{2} = g_{1} P_{{A_{1} }} /(g_{2} (K)^{{(1 - \alpha_{1} )}} P_{{A_{2} }} )\).
Apparently, Eq. 30 gives a maximum of the reaction rate as function of the equilibrium constant of the first step, as the dependence for activity changes from the order of α1 at low values of \(K_{1,t}^{*}\) to a negative value of α1 − 1 upon increasing \(K_{1,t}^{*}\).
Equation 30 can be also slightly modified to give dependence of the reaction rate for the optimal catalyst as a function of the Gibbs energy of the first step when the most abundant intermediate is formed:
Dependences from Eq. 30 are visualized in Figs. 2, 3, 4 and 5 showing valuations of the rate as a function of \(K_{1,t}^{*}\) depending the cluster size, parameter λ reflecting the range of surface non-uniformity (i.e. difference in the Gibbs energy of adsorption on terraces and edges), the Polanyi parameter α and the parameter p2.
Dependence of the reaction rate as a function of \(K_{1,t}^{*}\) according to Eq. 30 for λ = 10, p1 = 1 and Polanyi parameter equal to 0.5: a p2 = 3, b p2 = 10, c p2 = 30, d p2 = 50
Dependence of the reaction rate as a function of \(K_{1,t}^{*}\) according to Eq. 30 for λ = 10, p1 = 1, p2 = 10, for the Polanyi parameter equal to a 0.75, b 0.6, c 0.4, d 0.25
Dependence of the reaction rate as a function of \(K_{1,t}^{*}\) according to Eq. 30 for p1 = 1, p2 = 10, α = 0.5 and λ a 20, b 11, c 1, d − 10
Dependence of the reaction rate as a function of \(K_{1,t}^{*}\) according to Eq. 30 for p1 = 1, p2 = 10, α = 0.5 and λ a 20, b 11, c 1, d − 10
Fig. 2 demonstrates that the rates pass through maxima as expected as a function of the equilibrium constant of the first step in the reaction mechanism, i.e. binding the reactant to the catalyst surface. A higher value of the overall equilibrium constant K and in this sense larger changes in the Gibbs energy of the reaction result in a lower value of p2. As visible from Fig. 2 for such thermodynamically favorable reactions, the maxima in the rates are much broader being less dependent on the cluster size.
Larger values of p2 clearly make the second term in the denominator more prominent compared to unity thus resulting in a more pronounced asymptotic behavior as the rate in Eq. 30 starts to decrease according to \(r*\sim (K_{1,t}^{*} )^{{\alpha_{1} - 1}}\).
Smaller values of the Polanyi parameter (Fig. 3) make the maximum of the rate sharper. In the context of the optimum catalyst, it means that when the value of the Polanyi parameter is higher (e.g. equal to 0.6 as in Fig. 3b) for small active phase clusters, a broader range of potential catalysts can be active to a similar extent.
The influence of the surface nonuniformity manifested through a difference in the Gibbs energy of adsorption on terraces and edges is numerically explored in Fig. 4. Smaller values result in more narrow maxima, as could be anticipated. Obviously when the value of λ is close to zero, the curves almost coincide (Fig. 4c). Complete overlapping is achieved when λ = 0 (not shown). A negative value of λ correspond to an inversion of structure sensitivity (i.e. terraces are more catalytically active than edges). It follows from Fig. 4 that when structure sensitivity is less prominent (low values of λ) there are larger differences in the rates for the optimum and less optimal catalysts in comparison with reactions when structure sensitivity is more pronounced (Fig. 4a).
For the cases when reactions on edges are less efficient than on terraces, the influence of the overall thermodynamics (through parameter p2) and the Polanyi parameter is not that pronounced (Fig. 5) with narrow maxima in the rates as a function of \(K_{1,t}^{*}\).
In the considerations above the values of Polanyi parameters of steps were equal to each other while g1 and g2 were different. For the so-called symmetrical [7] irreversible reactions when not only α1 = α2, but also g1 = g2, it holds that:
When the ratio \(\frac{\alpha }{(1 - \alpha )} \approx 1\) and \(\alpha \approx 0.5\), Eq. 32 can be rearranged to:
Or, alternatively:
Equation 34 can be written in terms of the Gibbs energy of the first and second step:
Following an approach to obtain an approximate solution discussed in [7] the last term in Eq. 35 can be neglected giving:
For structure insensitive reactions, \(\lambda = 0\) holds and thus the Gibbs energy for formation of the intermediate is equal to the Gibbs energy of its decomposition. This result was reported in [7] for structure insensitive reactions and presented in [15] for chemical potentials. Obviously as the overall Gibbs energy of a two-step reaction (Fig. 1) is:
The Gibbs energy for formation of the intermediate
is thus related to the overall Gibbs energy, difference in adsorption thermodynamics on edges and terraces and the cluster size. Visualization of Eq. 38 is presented in Fig. 6.
An equation similar to Eq. 38 without the last term specific for structure sensitive reactions was reported in [24]. More recently [15], it was re-derived also for structure insensitive reactions using chemical potentials instead of the Gibbs energy.
Conclusions and outlook
Rational design of catalysts for two-step catalytic sequences is often based on the volcano plots relating activity with adsorption energy of the most abundant surface intermediate. The current treatment focuses on structure sensitive reactions, when activity depends on the cluster size of the active catalytic phase. The Gibbs energy for formation of the key intermediate was related in the current work to the overall Gibbs energy of a reaction, along with a difference in adsorption thermodynamics on edges and terraces and to the cluster size. Moreover, an expression for the reaction rate was derived, showing a connection between activity and the equilibrium constant for formation of the most abundant intermediate. Numerical analysis was performed to explore the impact on the overall equilibrium constant K, magnitude of surface nonuniformity and the value of Polanyi parameter on the reaction rate.
The concepts discussed in this work on the optimal binding energy defined through thermodynamics of the overall reaction can be used for the design of catalytic materials. An approach for the catalyst screening was for example described in [25] when the adsorption energy for a particular reaction was calculated based on DFT. Analysis in the current work indicates that the Gibbs energy for the formation of the key or the most abundant surface intermediate on the optimum catalyst is related not only to the overall thermodynamics, but also to the difference in adsorption between on edges and terraces and the cluster size. Obviously, a more detailed approach to structure sensitivity considerations can include also differences in adsorption characteristics of various crystallographic phases. For instance, in [13] peak positions in the volcano curves were calculated for hydrogen evolution reaction for various Pt species, showing clearly the difference between different crystal planes of Pt. Apparently for structure insensitive reactions, when the Gibbs energy of adsorption on poorly and highly coordinated metal sites is negligible, Eq. (38) is simply reduced to a half principle in the original form for the Gibbs energy [24] or a modified form for chemical potentials [14]. For the reactions when large metal/metal oxide clusters are involved a simplified approach can be also used for a rational catalyst screening. However, for design of catalytic materials for structure sensitive reactions when the reaction rate depends substantially on the cluster size, the latter should be considered during a computational screening. This is turn requires more efforts in describing properties of small nanosized clusters including for example interactions with the (metal oxide) supports [26].
References
Sabatier P (1920) La catalyse en chimie organique. Librairie Polytechnique, Paris et Liège
Balandin AA (1946) Zh Obshei Khimii 16:793
Temkin MI (1957) Zh Phys Khim 31:3
Medford AJ, Vojvodic A, Hummelshøj JS, Voss J, Abild-Pedersen F, Studt F, Bligaard T, Nilsson A, Nørskov JK (2015) J Catal 328:36–42
Tanaka K, Tamaru K (1963) J Catal 2:366
Sachtler WMH, Fahrenfort J (1960) Actes 2ème Congr Int Catal Paris 1:831
Temkin MI (1984) Kinet Catal 25:299
Boudart M, Tamaru K (1991) Catal Lett 9:15
Ichikawa S (1990) Chem Eng Sci 45:529–535
Temkin M (1979) Adv Catal 28:173
Murzin DYu, Salmi T (2016) Catal Kinet. Elsevier, Amsterdam
Murzin DYu (1994) React Kinet Catal Lett 53:467–474
Yang B, Burch R, Hardacre C, Headdock G, Hu P (2014) ACS Catal 4:182–186
Mao Y, Chen J, Wang H, Hu P (2015) Chin J Catal 36:1596–1605
Chen J-F, Mao Y, Wang H-F, Hu P (2019) ACS Catal 9:2633–2638
Kizuch S, Shaik S (2008) J Phys Chem A 112:6032–6041
Chen J, Chen Y, Li P, Wen Z, Chen S (2018) ACS Catal 8:10590–10598
Solel E, Tarannam N, Kozuch S (2019) Chem Commun 55:5306–5322
Boudart M (1969) Adv Catal 20:153
van Santen RA (2009) Acc Chem Res 42:57
Ligthart DAJM, van Santen RA, Hensen EJM (2011) J Catal 280:206
Murzin DYu (2010) J Catal 276:85
Murzin DYu, Parmon VN (2011) Catal Spec Period Rep RSC 23:179
Temkin MI (1986) Kinet Katal 57:533
Mao Y, Wang H-F, Hu P (2017) WIREs Comput Mol Sci 7:e1321
Rice PS, Hu P (2019) J Chem Phys 151:180902
Funding
Open access funding provided by Abo Akademi University (ABO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murzin, D.Y. On the optimum catalyst for structure sensitive heterogeneous catalytic reactions. Reac Kinet Mech Cat 131, 5–17 (2020). https://doi.org/10.1007/s11144-020-01835-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01835-3