Abstract
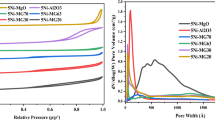
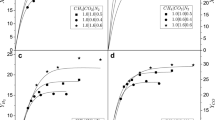
In the present work, appropriate reaction conditions were found to control the catalyst deactivation in the CO2 reforming of methane over Ni–Mg–Al catalyst. Experiments with variable CO2/CH4 ratio showed that the carbon deposition and the sintering of the catalyst decrease with increasing CO2/CH4 ratio. The carbon deposition was controlled at CO2/CH4 = 2, whereas a strong deactivation was observed for a ratio of CO2/CH4 < 1. The experiments with variable space time and fixed CO2/CH4 ratio showed that a shorter space time favors both the carbon deposition control and enhances the resistance of the catalyst to sintering. These results are supported by XRD, TPO and Raman Spectroscopy analyses. Catalyst presented high stability with the time-on-stream in the reaction at 700 °C, CO2/CH4 = 2, and space time of 1.2 g h/mol. The proper reaction conditions are suitable for high CO2 content feed.








Similar content being viewed by others
References
Gao Y, Jiang J, Meng Y et al (2018) A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers Manag 171:133–155. https://doi.org/10.1016/j.enconman.2018.05.083
Amin AM, Croiset E, Epling W (2011) Review of methane catalytic cracking for hydrogen production. Int J Hydrog Energy 36:2904–2935. https://doi.org/10.1016/j.ijhydene.2010.11.035
Abbas HF, Wan Daud WMA (2010) Hydrogen production by methane decomposition: a review. Int J Hydrog Energy 35:1160–1190. https://doi.org/10.1016/j.ijhydene.2009.11.036
Xu J, Zhou W, Li Z et al (2009) Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts. Int J Hydrog Energy 34:6646–6654. https://doi.org/10.1016/j.ijhydene.2009.06.038
Serrano-lotina A, Rodríguez L, Muñoz G et al (2011) Biogas reforming over La-NiMgAl catalysts derived from hydrotalcite-like structure: influence of calcination temperature. Catal Commun 12:961–967. https://doi.org/10.1016/j.catcom.2011.02.014
Priebe GPS, Kipper E, Gusmão AL et al (2016) Anaerobic digestion of chrome-tanned leather waste for biogas production. J Clean Prod 129:410–416. https://doi.org/10.1016/j.jclepro.2016.04.038
Deublein D, Steinhauser A (2011) Biogas from waste and renewable resources, Second. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim
Wang Y, Yao L, Wang Y et al (2018) Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst. ACS Catal 8:6495–6506. https://doi.org/10.1021/acscatal.8b00584
Zhou L, Li L, Wei N et al (2015) Effect of NiAl2O4 formation on Ni/Al2O3 stability during dry reforming of methane. ChemCatChem. https://doi.org/10.1002/cctc.201500379
Zain MM, Mohamed AR (2018) An overview on conversion technologies to produce value added products from CH4 and CO2 as major biogas constituents. Renew Sustain Energy Rev 98:56–63. https://doi.org/10.1016/j.rser.2018.09.003
Messaoudi H, Thomas S, Djaidja A et al (2018) Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J CO2 Util 24:40–49
Roy PS, Song J, Kim K et al (2018) CO2 conversion to syngas through the steam-biogas reforming process. J CO2 Util 25:275–282. https://doi.org/10.1016/j.jcou.2018.04.013
Pawar V, Appari S, Monder DS, Janardhanan VM (2017) Study of the combined deactivation due to sulfur poisoning and carbon deposition during biogas dry reforming on supported Ni catalyst. Ind Eng Chem Res 56:8448–8455. https://doi.org/10.1021/acs.iecr.7b01662
Nikoo MK, Amin NAS (2011) Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process Technol 92:678–691. https://doi.org/10.1016/j.fuproc.2010.11.027
De Rosa F, Smyth BM, McCullough G, Goguet A (2018) Using multi-criteria and thermodynamic analysis to optimize process parameters for mixed reforming of biogas. Int J Hydrog Energy 43:18801–18813. https://doi.org/10.1016/j.ijhydene.2018.08.127
Macario A, Fronteira P, Candamano S, Crea F, Luca P, Antonucci PL (2019) Nanostructured catalysts for dry reforming of methane. J Nanosci Nanotechnol 19:3135–3147. https://doi.org/10.1039/c5nj03268g
Cao P, Adegbite S, Wu T (2017) Thermodynamic equilibrium analysis of CO2 reforming of methane: elimination of carbon deposition and adjustment of H2/CO ratio. Energy Procedia 105:1864–1869. https://doi.org/10.1016/j.egypro.2017.03.546
Chein RY, Chen YC, Yu CT, Chung JN (2015) Thermodynamic analysis of dry reforming of CH4 with CO2 at high pressures. J Nat Gas Sci Eng 26:617–629
Arora S, Prasad R (2016) An overview on dry reforming of methane: strategies to reduce carbonaceous deactivation of catalysts. RSC Adv 6:108668–108688. https://doi.org/10.1039/c6ra20450c
Lavoie J (2014) Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front Chem 2:1–17. https://doi.org/10.3389/fchem.2014.00081
Abdulrasheed A, Jalil AA, Gambo Y et al (2019) A review on catalyst development for dry reforming of methane to syngas: recent advances. Renew Sustain Energy Rev 108:175–193. https://doi.org/10.1016/j.rser.2019.03.054
Ren HP, Song YH, Wang W et al (2015) Insights into CeO2-modified Ni-Mg-Al oxides for pressurized carbon dioxide reforming of methane. Chem Eng J 259:581–593. https://doi.org/10.1016/j.cej.2014.08.029
Calgaro CO, Perez-Lopez OW (2019) Biogas dry reforming for hydrogen production over Ni–M–Al catalysts (M = Mg, Li, Ca, La, Cu Co, Zn). Int J Hydrog Energy 44:17750–17766. https://doi.org/10.1016/j.ijhydene.2019.05.113
Zhang J, Wang H, Dalai AK (2007) Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J Catal 249:300–310. https://doi.org/10.1016/j.jcat.2007.05.004
Dahdah E, Rached JA, Aouad S et al (2017) CO2 reforming of methane over NixMg6-xAl2 catalysts : effect of lanthanum doping on catalytic activity and stability. Int J Hydrog Energy 42:12808–12817. https://doi.org/10.1016/j.ijhydene.2017.01.197
Kim AR, Lee HY, Cho JM et al (2017) Ni/M-Al2O3 (M=Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J CO2 Util 21:211–218. https://doi.org/10.1021/jp0367530
Habibi N, Wang Y, Arandiyan H, Rezaei M (2017) Effect of substitution by Ni in MgAl2O4 spinel for biogas dry reforming. Int J Hydrog Energy 42:24159–24168. https://doi.org/10.1016/j.ijhydene.2017.07.222
Casenave S, Martinez H, Guimon C et al (2001) Acid - base properties of Mg–Ni–Al mixed oxides using LDH as precursors. Thermochim Acta 379:85–93
Perez-lopez OW, Senger A, Marcilio NR, Lansarin MA (2006) Effect of composition and thermal pretreatment on properties of Ni–Mg–Al catalysts for CO2 reforming of methane. Appl Catal A Gen 303:234–244. https://doi.org/10.1016/j.apcata.2006.02.024
Sengupta S, Deo G (2015) Modifying alumina with CaO or MgO in supported Ni and Ni-Co catalysts and its effect on dry reforming of CH4. J CO2 Util 10:67–77. https://doi.org/10.1016/j.jcou.2015.04.003
Lino AVP, Assaf EM, Assaf JM (2018) NiMgAlCe catalysts applied to reforming of a model biogas for syngas production. Catal Lett 148:979–991. https://doi.org/10.1007/s10562-018-2304-9
Lucrédio AF, Assaf JM, Assaf EM (2014) Reforming of a model sulfur-free biogas on Ni catalysts supported on Mg(Al)O derived from hydrotalcite precursors: effect of La and Rh addition. Biomass Bioenergy 60:8–17. https://doi.org/10.1016/j.biombioe.2013.11.006
Li Z, Jiang B, Wang Z, Kawi S (2018) High carbon resistant Ni@Ni phyllosilicate@SiO2 core shell hollow sphere catalysts for low temperature CH4 dry reforming. J CO2 Util 27:238–246. https://doi.org/10.1016/j.jcou.2018.07.017
Cavani F, Trifirb F, Vaccari A (1991) Hydrotalcite-type anlonlc clays: preparation, properties and applications. Catal Today 11:173–301
Białas A, Mazur M, Natka P et al (2016) Hydrotalcite-derived cobalt—aluminum mixed oxide catalysts for toluene combustion. Appl Surf Sci 362:297–303
Hermes NA, Lansarin MA, Perez-lopez OW (2011) Catalytic decomposition of methane over M-Co–Al catalysts ( M 5 Mg, Ni, Zn, Cu). Catal Letters 141:1018–1025. https://doi.org/10.1007/s10562-011-0611-5
Escobar C, Perez-Lopez OW (2014) Hydrogen production by methane decomposition over Cu–Co–Al mixed oxides activated under reaction conditions. Catal Letters. https://doi.org/10.1007/s10562-014-1234-4
Calgaro CO, Perez-lopez OW (2017) Decomposition of methane over Co3-xAlxO4 (x = 0–2) coprecipitated catalysts : the role of Co phases in the activity and stability. Int J Hydrog Energy 42:29756–29772. https://doi.org/10.1016/j.ijhydene.2017.10.082
Zardin L, Perez-lopez OW (2017) Hydrogen production by methane decomposition over Co-Al mixed oxides derived from hydrotalcites : effect of the catalyst activation with. Int J Hydrog Energy 42:7895–7907. https://doi.org/10.1016/j.ijhydene.2017.02.153
Lu Y, Zhou P, Han J, Yu F (2015) Fischer-tropsch synthesis of liquid hydrocarbons over mesoporous SBA-15 supported cobalt catalysts. RSC Adv 5:59792–59803. https://doi.org/10.1039/C5RA10123A
Oliveira TKR, Rosset M, Perez-Lopez OW (2018) Ethanol dehydration to diethyl ether over Cu-Fe/ZSM-5 catalysts. Catal Commun 104:32–36. https://doi.org/10.1016/j.catcom.2017.10.013
Calgaro CO, Perez-Lopez OW (2019) Graphene and carbon nanotubes by CH4 decomposition over Co–Al catalysts. Mater Chem Phys 226:6–19. https://doi.org/10.1016/j.matchemphys.2018.12.094
Gousi M, Andriopoulou C, Bourikas K et al (2017) Green diesel production over nickel-alumina co-precipitated catalysts. Appl Catal A Gen 536:45–56. https://doi.org/10.1016/j.apcata.2017.02.010
Serrano-Lotina A, Rodríguez L, Muñoz G, Daza L (2011) Biogas reforming on La-promoted NiMgAl catalysts derived from hydrotalcite-like precursors. J Power Sources 196:4404–4410. https://doi.org/10.1016/j.jpowsour.2010.10.107
Clause O, Rebours B, Merlen E, Trifiró F, Vaccari A (1992) Preparation and characterization of nickel-aluminum mixed oxides obtained by thermal decomposition of hydrotalcite-type precursors. J Catal 133:231–246
Srivastava S, Jadeja GC, Parikh J (2017) Synergism studies on alumina-supported copper-nickel catalysts towards furfural and 5-hydroxymethylfurfural hydrogenation. J Mol Catal A Chem 426:244–256
de Souza G, Marcilio NR, Perez-Lopez OW (2014) Dry reforming of methane at moderate temperatures over modified Co-Al Co-precipitated catalysts. Mater Res 17:1047–1055. https://doi.org/10.1590/1516-1439.269614
Sikander U, Sufian S, Salam MA (2017) A review of hydrotalcite based catalysts for hydrogen production systems. Int J Hydrog Energy 42:19851–19868. https://doi.org/10.1016/j.ijhydene.2017.06.089
Liu H, Wierzbicki D, Debek R et al (2016) La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 182:8–16. https://doi.org/10.1016/j.fuel.2016.05.073
Serrano-lotina A, Martin AJ, Folgado MA, Daza L (2012) Dry reforming of methane to syngas over La-promoted hydrotalcite clay-derived catalysts. Int J Hydrog Energy 37:12342–12350
Jana P, de la Peña O’Shea VA, Coronado JM, Serrano DP (2011) Co-production of graphene sheets and hydrogen by decomposition of methane using cobalt based catalysts. Energy Environ Sci 4:778. https://doi.org/10.1039/c0ee00490a
Soldano C, Mahmood A, Dujardin E (2010) Production, properties and potential of graphene. Carbon N Y 48:2127–2150. https://doi.org/10.1016/j.carbon.2010.01.058
Zhang X, Wang F, Song Z, Zhang S (2019) Comparison of carbon deposition features between Ni/ZrO2 and Ni/SBA-15 for the dry reforming of methane. Reac Kinet Mech Cat. https://doi.org/10.1007/s11144-019-01707-5
Qiu Z, He D, Wang Y et al (2017) High performance asymmetric supercapacitors with ultrahigh energy density based on hierarchical carbon nanotubes@NiO core-shell nanosheets and defect-introduced graphene sheets with hole structure. RSC Adv 7:7843–7856. https://doi.org/10.1039/c6ra27369f
Los Santos Valladares LD, Ionescu A, Holmes S et al (2014) Characterization of Ni thin films following thermal oxidation in air. J Vac Sci Technol B 32:051808. https://doi.org/10.1116/1.4895846
Acknowledgements
The authors are grateful for the financial support provided by CAPES (Brazilian Agency for Improvement of Graduate Personnel) and CNPq (National Council of Science and Technological Development).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Calgaro, C.O., Rocha, A.L. & Perez-Lopez, O.W. Deactivation control in CO2 reforming of methane over Ni–Mg–Al catalyst. Reac Kinet Mech Cat 130, 159–178 (2020). https://doi.org/10.1007/s11144-020-01770-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01770-3