Abstract
“Support on support” type catalysts were developed by applying catalytic chemical vapour deposition (CCVD). Nitrogen-doped bamboo-like carbon nanotubes (N-BCNT) were synthetized on the surface of zeolite beads by using four different metals (Ni, Fe, Co and V). Based on the thermogravimetric analysis (TGA), the Ni, Co and Fe were catalytically active for nanotube production on the zeolite surface. It was confirmed by SEM measurements, that the surface of the beads was extensively covered by N-BCNTs. Based on the TGA and SEM results, two SoS systems were selected for further catalysts development, which differed the most in their nanotube content (difference 11.63 wt%) and surface coverage. These were two nickel containing samples with 6.72 and 18.35 wt% N-BCNT. Palladium nanoparticles were deposited (1 wt%) on the surface of the N-BCNT/zeolite systems, and the final Pd/SoS catalysts were tested in hydrogenation of 1-butene. The conversion maximum (X = 100%) was reached after 6 min in both cases independently of the nanotube content and coverage (WHSV: 5.49). To measure the lifetime of the selected catalysts, the WHSV was increased from 5.49 to 11 (by increasing the gas flow) and the systems were still active after 6 h. Thus, the lifetime of the catalysts is longer than 6 h. Furthermore, by increasing the gas flow (WHSV = 11), higher conversion was achieved (98%) with the sample which has a higher nanotube content Therefore, smaller carbon nanotube content is still as catalyst support. The final Pd/SoS catalysts were active, easy to treat and removable from the reactors and could work with smaller carbon nanotube content as well. Thus, a more economical catalysts was developed, than a solely carbon nanotube-based counterparts which lack the zeolite core.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different porous materials, such as zeolite, silica or activated carbon forms are regularly applied as catalyst supports in hydrogenation processes [1,2,3,4]. The mentioned support materials contain several micropores, thereby their surface areas are large. However, this feature, the presence of micropores in a catalytic system could limit the diffusion and, therefore, lower the reaction rates of the processes [5]. Furthermore, the catalytically active metal particles could be adsorbed in the micropores and thus, the accessible metal content of the catalyst could be decreased considerably. If other support materials such as carbon nanotubes (CNTs) are also included into the catalytic system besides the porous substrates (pellets, rings or beads), the above-mentioned problems can be reduced or avoided [6]. In this case, “support on support” (SoS) type catalysts are prepared which contain a “core” material (zeolite beads, pellets or others) support and carbon nanotubes as primary support for the catalytically active metal particles.
Carbon nanotubes are excellent catalyst supports, thanks to their extraordinary properties, such as high surface area, nanostructure, thermal stability, outstanding mechanical and electronic properties [7,8,9]. Carbon nanotube supported catalysts were successfully applied in hydro-desulfurization [10], hydro-formylation [11], Fischer–Tropsch synthesis [12], and oxidation [13]. The nanotubes were also effective support materials in hydrogenation reactions of various compounds including butene [14], ethyl-benzene [15], nitro-benzene [16], nitro-cyclohexane [17], and methyl-styrene [18]. The properties of CNTs can be further enhanced by incorporating nitrogen atoms into their structure which will led to the so-called nitrogen-doped bamboo-like carbon nanotubes (N-BCNTs). The structure of the N-BCNTs are altered compare to their nitrogen free counterparts. Further adsorption sites appear thanks to the incorporated nitrogen atoms, which will increase the potential interactions with the catalytically active metals and led to the formation of more effective heterogeneous catalysts [19,20,21]. However, N-BCNTs has high colloid stability in aqueous phase, because of the several dissociable functional groups in their structure and the graphene edges caused by the nitrogen atoms. Thus, the recovery of solely N-BCNT based catalysts is difficult [22]. But the problem can be handled by using additional support materials for the N-BCNTs in a “SoS” system, as it was mentioned above. To design an ideal catalytic system for hydrogenation, “SoS” catalysts were developed based on zeolite beads supported N-BCNTs which were decorated by catalytically active metal (Pd). The activity of different Pd/SoS were compared and the effect of the N-BCNT content and coverage was studied.
Experimental
Materials and methods
The nitrogen-doped bamboo-like carbon nanotubes were synthesized by employing the CCVD (Catalytic Chemical Vapour Deposition) process with n-butylamine (WVR) as carbon source. The catalysts were prepared by using one of the following metal salts, nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O, Reanal), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, Reanal), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, Reanal), or ammonium metavanadate (NH4VO3, Reanal), in combination with Grace MS 5A zeolite beads (Roth) and Patosolv (mixture of aliphatic alcohols, WVR). The chemical composition of the applied zeolite is Ca/nNa12-2n[(AlO2)12(SiO2)12] ·H2O. The pore size and the size of the beads are ~ 5Å and 1.6-2.5 mm, respectively. Pd(NO3)2·2H2O (Reanal) was used to deposit the catalytically active Pd nanoparticles onto the surface of the N-BCNTs. 1-butene (purity: 99.5%), hydrogen (purity: 99.999%) and nitrogen (purity: 99.995%) from Messer was applied to compare the activity of the catalysts in hydrogenation.
Synthesis of the SoS catalysts
The first step of the catalyst development was the impregnation of the zeolite beads with the transition metal salts. The zeolite beads were added to the aqueous solution of iron, cobalt, nickel and vanadium salts and the metal content was kept to 2 wt% in each case (Table 1). Thereafter the water was evaporated by using vacuum rotary evaporator. All samples were dried at 105 °C overnight.
The impregnated zeolite beads were used as catalysts in the CCVD method to synthetize N-BCNTs. In each case, 2 g catalyst was placed in a quartz reactor and the synthesis were carried out for 30 min. The carbon source was butylamine, which was added by a syringe pump (6 ml/h). Four different temperatures (650, 700, 750 and 800 °C) were tested to select the optimal conditions. The efficacy of the procedures was compared based on the amount of the synthetized carbon nanotube.
In the final step of the catalyst development, palladium nanoparticles were deposited onto the surface of SoS beads. The N-BCNT coated zeolite (1.2 g) was impregnated by 1.5 ml 2 wt% palladium solution (1 g Pd(NO3)2·2H2O in 50 ml water) which was added to 30 ml distilled water. The water was evaporated and the Pd impregnated SoS beads were dried at 120 °C overnight. The impregnated beads were heat-treated in nitrogen flow at 400 °C for 20 min. Then, to form catalytically active Pd nanoparticles on the surface of the beads, the system was hydrogenated at 400 °C for 30 min. The appearance of the palladium nanoparticles on the catalyst surface was confirmed by SEM–EDS measurements.
Characterization techniques
The presence and dispersity of N-BCNTs on the surface of the SoS samples were examined by Hitachi S4800 scanning electron microscopy (SEM). All SoS beads were glued onto the Carbon Tape surface. The N-BCNT content of the samples was measured by thermogravimetric analysis by using a Netzsh TG Tarsus instrument. The heat rate was 10 °C/min and the measurements were carried out in oxidative atmosphere (N2/O2 mixture, 14 ml/min/6 ml/min, respectively).
Catalytic study of the SoS catalyst samples
The catalytic activity of the samples was followed by a Bruker Vertex 70 Fourier transform infrared spectroscope, which was equipped with a gas cell. The atmospheric hydrogenation reactor system was connected to the gas cell in the infrared spectroscope (Fig. 1a–h). Nitrogen (40 ml/min), hydrogen (40 ml/min) and 1-butene (20 ml/min) was added to the system at atmospheric pressure. The hydrogen/1-butene molar ratio was 2:1. The temperature in the reactor was 50 °C and 0.5 g SoS catalyst sample was used during all measurements. The weight hour space velocity (WHSV) was 5.49 kg 1-butene/1 kg catalyst/1 h.
Experimental setup to measure the catalytic activity of the developed “support on support” (SoS) catalysts in gas phase hydrogenation: a reactor with the catalyst in tube furnace, b, c FTIR instrument connected to a computer, d, e temperature indicator controller (TIC) with thermo element, f four-way valve for gas inlet into the reactor and the FTIR gas cell, g mass flow meter and controller, h valves for gases
The absorption band of the C=C stretching vibration mode at 1646 cm−1 was used to follow the hydrogenation of 1-butene. The system was calibrated for five different 1-butene concentrations to ensure a properly linear quantitative response.
The efficacy of the catalytic hydrogenation was measure by the conversion (X%) of 1-butene based on the following equation (Eq. 1):
Results and discussion
Characterization of the “Support on Support” beads
The presence and dispersibility of nitrogen-doped carbon nanotubes on the surface of the zeolite was studied by SEM. The nanotubes can be clearly seen on the SEM pictures and their amount depend on the synthesis temperature and the metal used for impregnating the zeolite beads. The zeolite beads which were impregnated with cobalt, iron or nickel were effective in nanotube synthesis (SI, Fig. S1). Several carbon nanotubes can be located on the surface of these samples. However, the vanadium containing zeolite samples did not show catalytic activity and on the surface of these beads carbon nanotubes were not formed (SI, Fig. S1).
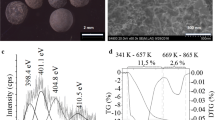
The SEM results were confirmed by thermogravimetric measurements. The N-BCNT content of the samples prepared at different temperatures was defined by using the TG-DTG results (SI, Fig. S2). The carbon nanotube containing of the SoS catalysts can be seen in the Table 2.
The inactivity of the vanadium containing samples during the CCVD procedure was striking. The nanotube content was less than 3.5 wt% at 750 °C, and even less at other temperatures. On the surface of the iron containing zeolite beads 6.55 wt% N-BCNT was deposited at 750 °C, which was the optimal condition as by decreasing or increasing the temperature the nanotube content was smaller. The nickel containing samples were quite effective and 18.35 wt% nanotubes formed at 650 °C. The best sample in terms of nanotube formation was the one with 2 wt% cobalt at 650 and 700 °C. In this case, ~ 20 wt% N-BCNT was deposited on the surface of the beads. Three significant steps can be seen on the TG curves. The mass loss from ~ 350 to ~ 600 °C can be attributed to the ignition of carbon nanotubes. The initial temperature of the thermal oxidation of nanotubes in the case of each samples are different, owing to the different catalytic effect of the metals. Low ignition temperature (353 °C) was measured in the case of Co containing samples. The other two steps on the TG curves derives from the elimination of the adsorbed water and different organic compounds which formed during the decomposition of the carbon source of the nanotube synthesis (n-butylamine). Heat treatment was applied to remove these adsorbed components during the final Pd/SoS catalyst preparation procedure.
Based on the SEM pictures and the TGA results, two Ni containing N-BCNT/zeolite samples (650 and 800 °C) were chosen for further catalyst preparation. The nanotube content and coverage of the two samples varied the most (6.72 and 18.35 wt%) within the prepared set of zeolite supported N-BCNTs. Thus, it can be tested whether more nanotube (a thick nanotube layer) is necessary or not to achieve high catalytic activity. Palladium nanoparticles were deposited onto the surface of the selected N-BCNT/zeolite beads, and the activity of the final Pd/SoS catalysts were tested using 1-butene hydrogenation as a test reaction.
Characterization of the Pd/SoS catalysts
The presence of palladium nanoparticles on the surface carbon nanotubes of the SoS catalysts was confirmed by SEM (Fig. 2a, b). The particle diameters were measured with the ImageJ program (Fig. 2c, d). The Pd particle size distribution on the surface of the two SoS catalysts is very similar. The mean particle diameter was 10.2 nm and 11.6 nm, for the 6.72 wt% and for the 18.35 wt% N-BCNT containing sample, respectively. 80% of the particles were between 4 and 14 nm on the surface of the 6.72 wt% nanotube sample (Fig. 2c). In the case of the 18.35 wt% nanotube containing catalyst, most of the Pd particles (80%) were between 6 and 13 nm (Fig. 2d). A small difference can be observed in the case of the maximum diameters as well (16 and 20 nm), but apart from that, the Pd particles are not substantially different in the two catalysts. On the EDS spectrum several peaks can be identified, which can be attributed to Pd and other compounds of the zeolite beads (Fig. 2e, f).
Catalytic tests of the Pd/SoS catalysts
Based on the catalytic tests, the butene conversion depends on the hydrogenation time (eq. 1). The conversion maximum (X = 100%) was reached after 6 min in both cases (Fig. 3a). The developed catalysts independently from their nanotube content were able to catalyse the hydrogenation process with maximum activity of 5.49 WHSV. The moles of hydrogenated butene per 1 mol palladium per min was calculated after 6 min hydrogenation. The amount of the hydrogenated 1-butene was 4.91 mol achieved by 1 mol palladium within 1 min in case of the 6.72 wt% N-BCNT containing catalyst. By using the 18.35 wt% nanotube containing sample, the 1-butene consumption was somewhat lower 4.33 mol.
To measure the lifetime of the selected catalysts, the WHSV was increased up to 11 (by increasing the gas flow) and the systems were still active after 6 h. Thus, the lifetime of the catalysts is longer than 6 h. Furthermore, by increasing the gas flow (WHSV = 11), higher conversion was achieved (98%) with the sample which has a higher nanotube content (Fig. 3b).
Conclusion
Palladium containing “support on support” type catalysts were developed for catalytic hydrogenation processes. The primer catalyst supports were the N-doped bamboo-like carbon nanotubes (N-BCNTs) which were deposited on the surface of zeolite beads used as the “core” of the system. The zeolite beads were impregnated with four different metals (Co, Fe, Ni and V) which were used as catalysts to deposit N-BCNTs onto the surface of the beads by using the CCVD method at different temperatures. The N-BCNT content of the system was measured by TGA and studied by SEM. Based on the TGA results, two Ni containing N-BCNT/zeolite samples (650 and 800 °C) were chosen for further catalyst preparation. The nanotube content and coverage of the two samples varied the most (6.72 and 18.35 wt%) within the prepared set of zeolite supported N-BCNTs. On the surface of these samples, palladium nanoparticles were deposited (1 wt%), and the final Pd/SoS catalysts were tested in hydrogenation of 1-butene. The conversion maximum (X = 100%) was reached after 6 min hydrogenation in both cases. Catalytic activities of the two samples were compared based on following values: 4.91 mol 1-butene was hydrogenated on 1 mol Pd per 1 min in case of the 6.72 wt% N-BCNT contained catalyst. By using of the 18.35 wt% nanotube contained sample was reached 4.33 mol 1-butene consumption per 1 mol Pd per min, by applying of 5.49 WHSV.
The lifetime of the catalysts was measured and it was found to be longer than 6 h. During the lifetime tests the WHSV was increased from 5.49 to 11 (increased gas feed), and it led to different 1-butene conversions in case of the two catalysts. Higher 1-butene feed resulted different activity in case of both catalysts, despite the same Pd content and particle size. This can be associated with the different N-BCNT content of the catalysts and it shows that higher carbon nanotube content increased the catalytic activity of the samples (from 86 to 98%). The final Pd/SoS catalysts are active, easy to treat and removable from the reactors and could work with smaller carbon nanotube content as well. Therefore, these catalysts are more economical, than their solely carbon nanotube-based counterparts which lack the zeolite core.
References
Teh LP, Triwahyono S, Jalil AA, Mamat CR, Sidik SM, Fatah NAA, Mukti RR, Shishido T (2015) Nickel-promoted mesoporous ZSM5 for carbon monoxide methanation. RSC Adv 79:64651–64660
Yung MM, Starace AK, Mukarakate C, Crow AM, Leshnov MA, Magrini KA (2016) Biomass catalytic pyrolysis on Ni/ZSM-5: effects of nickel pre-treatment and loading. Energy Fuel 30:5259–5268
Pudukudy M, Yaakob Z, Akmal ZS (2015) Direct decomposition of methane over Pd promoted Ni/SBA-15 catalysts. Appl Surf Sci 353:127–136
Muradov N, Smith F, T-Raissi A (2005) Catalytic activity of carbons for methane decomposition reaction. Catal Today 102–103:225–233
Mahata N, Soares OSGP, Rodríguez-Ramos I, Pereira MFR, Órfão JJM, Figueiredo JL (2013) Promotional effect of Cu on the structure and chloronitrobenzene hydrogenation performance of carbon nanotube and activated carbon supported Pt catalysts. Appl Catal B 464–465:28–34
Vanyorek L, Bánhidi O, Muránszky G, Sikora E, Prekob Á, Viskolcz B (2018) Chlorate elimination by catalytically hydrogenation, catalyst development and characterization. Catal 125:583–593
Collins PG, Arnold MS, Avouris P (2001) Engineering carbon nanotubes and nanotube circuits using electrical breakdown. Science 292:706–709
Yakabson BI, Smalley RE (1997) Fullerene Nanotubes: C1,000,000 and beyond. Am Sci 85:324–337
Rouff RS, Lorents DC (1995) Mechanical and thermal properties of carbon nanotubes. Carbon 33:925–930
Yu Z, Fareid LE, Moljord K, Blekkan EA, Walmsley JC, Chen D (2008) Hydrodesulfurization of thiophene on carbon nanofiber supported Co/Ni/Mo catalysts. Appl Catal B 84:482–489
Zhang Y, Zhang HB, Lin GD, Chen P, Yuan YZ, Tsai KR (1999) Preparation, characterization and catalytic hydroformylation properties of carbon nanotubes-supported Rh-phosphine catalyst. Appl Catal A 187:213
Bahome MC, Jewell LL, Hildebrandt D, Glasser D, Coville NJ (2005) Fischer-Tropsch synthesis over iron catalysts supported on carbon nanotubes. Appl Catal A 287:60–67
Ovejero G, Sotelo JL, Rodriguez A, Diaz C, Sanz R, Garcia J (2007) Platinum catalyst on multiwalled carbon nanotubes for the catalytic wet air oxidation of phenol. Ind Eng Chem Res 46:6449
Vanyorek L, Kristály F, Mihalkó A, Bánhidi O, Kónya Z, Kukovecz Á, Lakatos J (2015) Synthesis and 1-butene hydrogenation activity of platinum decorated bamboo-shaped multiwall carbon nanotubes. Reac Kinet Mech Cat 116:371–383
Chambers A, Nemes T, Rodriguez NM, Baker RTK (1998) J Phys Chem B 102:2251
Prekob Á, Muránszky G, Hutkai ZSG, Pekker P, Kristály F, Viskolcz B, Vanyorek L (2018) Hydrogenation of nitrobenzene over a composite catalyst based on zeolite supported N-doped carbon nanotubes decorated with palladium. Reac Kinet Mech Cat 125:583
Serp P, Corrias M, Kalck P (2003) Carbon nanotubes and nanofibers in catalysis. Appl Catal A 253:337–358
Pham-Huu C, Kelle N, Ehre G, Charbonniere LJ, Ziessel R, Ledoux MJ (2001) Carbon nanofiber supported palladium catalyst for liquid-phase reactions: an active and selective catalyst for hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. J Mol Catal A 170:155
Ibrahim EMM, Khavrus VO, Leonhardt A, Hampel S, Oswald S, Rümmeli MH, Büchner B (2010) Synthesis, characterization, and electrical properties of nitrogendoped single-walled carbon nanotubes with different nitrogen content. Diamond Relat Mater 19:1199–1206
Li YH, Hung TH, Chen YH (2009) A first-principles study of nitrogen- and boron-assisted platinum adsorption on carbon nanotubes. Carbon 47:850–855
Ayala P, Arenal R, Rümmeli M, Rubio A, Pichler T (2010) The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 48:575–586
Vanyorek L, Sikora E, Kiss A, Sike Á, Hutkai ZSG, Pekker P, Viskolcz B, Fiser B (2018) Nitrogen-doped bamboo-shaped carbon nanotube supported catalysts for heterogeneous hydrogenation—the effect of surface polarity. Reac Kinet Mech Cat 125:37–46
Acknowledgements
Open access funding provided by University of Miskolc (ME). This research was supported by the European Union and the Hungarian State, co-financed by the European Regional Development Fund in the framework of the GINOP-2.3.4-15-2016-00004 project, aimed to promote the cooperation between the higher education and the industry. Further support was provided within the framework of the EFOP-3.6.1-16-2016-00011 “Younger and Renewing University – Innovative Knowledge City – institutional development of the University of Miskolc aiming at intelligent specialisation” project which is implemented within the Széchenyi 2020 program and funded by the European Union, co-financed by the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vanyorek, L., Prekob, Á., Baráth, M. et al. Development of nitrogen-doped bamboo-like carbon nanotubes coated zeolite beads as “support on support” catalyst for the catalytic hydrogenation of olefins. Reac Kinet Mech Cat 127, 705–714 (2019). https://doi.org/10.1007/s11144-019-01592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01592-y