Abstract
Cefquinome sulfate is a veterinary, parenteral, fourth-generation cephalosporin with a methoxyimino–aminothiazolyl moiety into the acyl side chain and the quaternary quinoline group at position 3 of the cefem ring. Cefquinome sulfate is known to undergo degradation, which could be additionally increased by the presence of some critical factors such as temperature, relative air humidity, buffer components as well as hydrogen and hydroxide ions. The aim of this study was to evaluate the factors underlying the stability of cefquinome sulfate (CFQ) in aqueous solution and in the solid state taking into account general and specific acid–base hydrolysis in the pH range 0.45–10.48 at 343 K; the effect of temperature on the stability at 0% RH and an increased relative air humidity (RH = 76.4%); the influence of relative air humidity at 343 K (66.5–90%). To determine the observed rate constants, an isocratic HPLC–UV method was used. The three pKa values of cefquinome sulfate, kinetic (kobs., kpH), and activation parameters (Ea, ∆H≠, and ∆S≠) were determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fourth-generation cephalosporins have a broad spectrum of antibacterial activity against Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa and Enterobacteriaceae. Those compounds are also easily transported across the blood–brain barrier [1,2,3,4]. Fourth-generation cephalosporins are used to treat infections of the urinary tract, lungs, skin and soft tissues as well as in post-operative prophylaxis [3]. A beta-lactam group of cephalosporin and all beta-lactam antibiotics are essential for their antibacterial activity. They block the –CH2OH group of biocatalysts (transpeptidase, carboxypeptidase and endopeptidase) irreversibly, and thus inhibit bacterial cell wall biosynthesis [5]. Degradation products of beta-lactam antibiotics are generally inactive and are more allergenic than the parent compounds. The degradation of beta-lactam group may be caused or catalyzed by environmental factors such as the concentration of hydrogen ion, the components of the buffer, the concentration of neutral salts or light in aqueous solutions and the relative air humidity and the temperature in the solid phase.
Cefquinome sulfate is a veterinary, parenteral, fourth-generation cephalosporin. A significant enhancement of activity and an extension of the antibacterial spectrum were achieved by the introduction of a methoxyimino–aminothiazolyl moiety into the acyl side chain of the cephalosporin, which made it resistant to inactivation by β-lactamases [6,7,8,9,10,11]. The quaternary quinoline group at position 3 in the cefem ring and the resulting zwitterionic structure facilitate the rapid penetration of cefquinome across the biological membranes of animals and bacteria, ensuring its fast bactericidal action after injection.
It is active against the majority of strains found in animal infections such as Actinobacillus spp., Haemophilus spp., Clostridium spp., Corynebacterium, Erysipelohtrixthusiopathie, Proteus spp., Salmonella spp., Streptococcus spp., Pasteurella spp., Staphylococcus spp., Pseudomonas aeruginosa, Escherichia coli and Enterobacteriaceae. It is also active against many strains of methicillin-resistant staphylococci and enterococci. Cefquinome sulfate is often applied in the treatment of meningitis-mastitis-agalactie (MMA) [8,9,10,11].
HPLC [12] and colorimetric methods [13] for the determination of cefquinome sulfate in bulk and dosage form were developed. Shantier’s studies showed that cefquinome sulfate solutions are degraded via hydrolysis, which appears to depend on temperature and hydroxide ion concentration [13]. Chromatographic methods were used for the analysis of cefquinome sulfate in animal biological fluids and tissues [14,15,16]. The HPLC and TLC methods developed in this study were successfully used to separate the alkaline degradation products from the parent compound [17]. Also the stability of cefquinome sulfate in intravenous solutions was studied [12].
The aim of this study is an evaluation of the stability of CFQ in aqueous solutions and in solid state using an isocratic HPLC-UV method to determine the observed rate constants. The studies in aqueous solutions included the influence of general and specific acid–base hydrolysis of cefquinome sulfate in the pH range 0.45–10.48 at 343 K. In a study in the solid state, the effect of temperature on the stability of CFQ at increased relative air humidity (0 and 76%) and the influence of relative air humidity at 343 K (66.5–90%) was studied.
Experimental
Materials and methods
Cefquinome sulfate (CFQ) (Fig. 1) (Bepharm Ltd., China) is a white, crystalline powder, soluble in water, slightly soluble in ethanol, insoluble in diethyl ether and chloroform. All other chemicals and solvents were obtained from Merck KGaA, Germany, and were of analytical or high-performance liquid chromatographic grade. High-quality pure water was prepared by using the Millipore purification system (Millipore, Milsheim, France, model Exil SA 67120).
Determination of pKa values (potentiometric titration)
The pH measurements were made with a Mettler-Toledo DL 50 titrator equipped with a Mettler-Toledo DG 111 SC glass-combined electrode. The calibration was performed with standard buffer solutions with pH 4.01, 7.00 and 9.21 (Hamilton, Switzerland). The standard potential, slope and zero point of the electrode were established according to the electrode maintenance instructions. The solutions of sodium hydroxide (0.1 M) and hydrochloric acid (0.1 M) were standardized against standard potassium hydrogen phthalate (Merck, Germany) and tris(hydroxymethyl)-aminomethane (Merck, Germany).
The solution of CFQ was prepared by dilution of 0.12 g of the substance in 40.0 mL of deionized water. The samples were titrated with sodium hydroxide (0.1 M). The titration was performed in the pH range 2.7–11.5 under constant ionic strength (µ = 0.1 M) and under gaseous nitrogen atmosphere at 22 °C (295 K).
In order to establish the pKa values of CFQ three potentiometric titrations were performed and Hyperquad least-squares calculations were conducted. When the best possible fit was achieved, the non-negative non-linear least-squares calculation was used for the final calculation cycle [18].
Chromatographic conditions
Chromatographic separation and quantitative analysis were performed by using an HPLC method [12]. The analytical system consisted of a Shimadzu SPD-20A Prominence UV/VIS detector, a Rheodyne with a 50 µL loop. A LiChrospher RP-18 column (250 mm × 4.6 mm, 5 µm particle size, Merck, Germany) was used as the stationary phase. The mobile phase consisted of a mixture of 10 volumes of acetonitrile and 90 volumes of phosphate buffer (0.02 M, pH = 7.0). The flow rate was 1.0 mL/min and UV detection was performed at 268 nm. Although the method was evaluated and validated for the stress degradation study, the selectivity was examined during a stability study of CFQ [12].
Kinetic procedures
The degradation of CFQ in aqueous solutions was studied at 343 K in the pH range 0.45–10.48. The pH values of the reaction solutions and those of the buffer standards were measured at reaction temperature. The pH values of the reaction solutions in HCl were calculated from the equation pH = −log fHCl [HCl]. The activity coefficient fHCl was obtained or calculated from the literature data [19]. The ionic strength of all the solutions was adjusted to 0.50 M with a solution of sodium chloride (4 M). Solutions of the desired pH and ionic strength of 0.50 M were heated to 343 K; than a sample of CFQ was added. The initial concentration of CFQ was 0.2 mg/mL. At selected times, determined by the rate of degradation, samples of the solutions (1.0 mL) were collected and instantly cooled with a mixture of ice and water. Samples with pH above 7.5 were neutralized by using HCl solutions at concentrations ensuring that their pH was approximately 2.
For the forced aging test in the solid state, 5.0 mg samples of CFQ were weighed into 5 mL vials. To evaluate their stability at increased relative air humidity, they were placed in heat chambers at 333, 343, 353 and 363 K in RH 76.4% and at 343 K (RH 66.5–90.0%). To evaluate the stability of CFQ in dry air, the vials were immersed in a sand bath placed in heat chambers at 363, 373, 383 and 393 K. At specified time intervals, determined by the rate of degradation, the vials were removed, cooled at room temperature, and their contents were dissolved in distilled water. So obtained solutions were quantitatively transferred into measuring flasks and diluted with the same solvent to 25.0 mL.
Onto the column, 50 µL of samples of the solutions were injected.
Results and discussion
Three different dissociation constants were established, with the first protonation constant (pKa 2.75) corresponding to the carboxylic group, the second (pKa 3.75) to the primary amine group of the thiazole ring and the third (pKa 7.54) to the tertiary amine group of the quinoline ring.
The HPLC method with DAD detection used in this study has been found suitable for the determination of cefquinome sulfate under the stress conditions of hydrolysis, oxidation, photolysis and thermal degradation [20]. In this study the selectivity of the HPLC method in the presence of CFQ degradation products was confirmed. In the chromatograms, the symmetrical peak of analyzed substance (tR, 8.54 min) was well separated from the peak of the degradation products (tR, 5.71; 16.94 min) (Fig. 2).
Observed rate constants
The kinetic model of CFQ degradation in aqueous solutions and in the solid state was established by analyzing the percentage of the remaining drug concentration in the samples to be studied after their exposure to the predefined stress conditions. In each experiment the gradual decrease of CFQ concentration with time occurred. Each semilogarithmic plot c = f(t) was linear. The mathematical procedure of model-fitting confirmed this observation, indicating that under the conditions to be applied in our experiment, CFQ degrades according to a pseudo-first order reaction. Hence, the following equation was used in order to determine the appropriate kinetic parameters:
Here ct and c0 are the time-dependent concentration and the initial concentration of CFQ at time t > 0 and t = 0, respectively, kobs is the observed rate constant of the pseudo-first order reaction of CFQ degradation. The number of measurements of ct for each series ranged from 8 to 12. For the interpretation of the straight curves y = ax + b the following statistical parameters of the respective equation were calculated by means of the least square method: a ± Δa; b ± Δb, standard errors (Sa, Sb, Sy) and the coefficient of linear correlation r. The values of Δa and Δb were calculated for f = n – 2, with α = 0.05.
Buffer catalysis
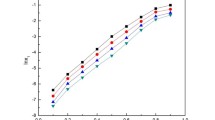
At a constant pH, ionic strength and temperature, the rate constants (kobs) for the degradation of CFQ increased linearly as the total concentrations of the phosphate (pH 1.54–2.95), acetate (pH 3.66–5.47) and borate (pH 7.52–10.48) buffers increased (Fig. 3). When some buffer components catalyze the degradation, the observed rate constants are a sum of catalytic rate constants of the buffer components and the rate constants at zero buffer concentration (general acid–base catalysis). Under the condition of general acid–base catalysis the kobs were calculated from the following equation:
Here [B]T is the total buffer concentration, kpH is the rate constant at zero buffer concentration, kB is a composite rate constant that includes the catalytic effects of the buffers.
The catalytic effect of the acetate buffer was investigated at pH 3.66–5.47. The observed rate constants were calculated from the following equation:
Here AcH is the undissociated acetate acid and Ac– is the acetate ion. The total acetate concentration BT, is:
The observed rate constant was calculated from the pKa of the acetic acid (4.52) and Eqs. 3 and 6.
From the slopes of the plots kobs versus (BT) for each pH, the buffer catalytic rate constants were calculated.
The buffer catalytic effect of phosphate (pH 1.54–2.95, pKa 2.10) and borate (pH 7.52–10.48, pKa 8.90) buffers were calculated from the following equations:
From Eqs. 6 to 8, the kpH values and the buffer catalytic rate constants of the phosphate, acetate, borate buffers components were calculated (Table 1).
Under the conditions of this study, the rate constants (kobs) did not depend on the total concentrations of the phosphate (pH 5.0–6.82) buffer, which indicated that the components of this buffer (KH2PO4, Na2HPO4) did not catalyze the degradation of CFQ. To verify that the differences between kobs determined at different buffer concentrations were not statistically significant the parallelism test was used. Since in the reaction solutions of CFQ in HCl, phosphate (pH 5.76–6.82) buffer as well as in NaOH general acid–base catalysis was not observed, in those solutions kobs = kpH.
pH-rate profiles
The rate constants kpH determined in hydrochloric acid, sodium hydroxide, phosphate buffer (pH 5.76–6.82) and under the conditions of general acid–base catalysis were used to calculate the relationship log kpH = f(pH) (Fig. 4). The semilogarithmic relationship kpH–pH indicated that in water solutions at pH 0.45–10.48 the following reactions occurred:
-
hydrolysis of di- and monocations of CFQ catalyzed by hydrogen ions (\({\text{k}}_{{{\text{H}}^{ + } }}\) and \({\text{k}^{\prime}}_{{{\text{H}}^{ + } }}\)),
-
spontaneous hydrolysis of zwitter ions and monoanions of CFQ under the influence of water (\({\text{k}}_{{{\text{H}}_{2} {\text{O}}}}\) and \({\text{k}^{\prime}}_{{{\text{H}}_{2} {\text{O}}}}\)),
-
degradation of CFQ molecules catalyzed by hydroxide ions (\({\text{k}}_{{{\text{OH}}^{ - } }}\)).
The total reaction rate was equal to the sum of partial reaction rates:
\({\text{a}}_{{{\text{H}}^{ + } }}\) and \({\text{a}}_{{{\text{OH}}^{ - } }}\) are the hydrogen and hydroxide ions activities, respectively, f1–f4 are the fractions of the molecules of CFQ (di- and monoprotonated molecules, zwitter ions and monoanions). The values of f1–f4 were calculated from the values of pKa of CFQ, which were 2.75 and 3.75 and 7.54 at 295 K (Fig. 5).
The catalytic rate constant \({\text{k}}_{{{\text{OH}}^{ - } }}\) was calculated from the plot kpH = f(\({\text{a}}_{{{\text{OH}}^{ - } }}\)) using kpH values from the pH range above 6. The plot kpH = f(\({\text{a}}_{{{\text{OH}}^{ - } }}\)) is linear with a positive slope that was equal to \({\text{k}}_{{{\text{OH}}^{ - } }}\) (Fig. 6).
The catalytic rate constants of the spontaneous hydrolysis of CFQ molecules under the influence of water were calculated from the pH range above 6, where f3 + f4 → 1 from the following equation:
The plot \({\text{k}^{\prime}}_{\text{pH}}\) = f(f4) was linear and the catalytic rate constant \({\text{k}^{\prime}}_{{{\text{H}}_{2} {\text{O}}}}\) is equal to the value of \({\text{k}^{\prime}}_{\text{pH}}\) for f4 = 1 (Fig. 7). The catalytic rate constant \({\text{k}}_{{{\text{H}}_{2} {\text{O}}}}\) was calculated as the mean value from the equation:
The kpH value from pH range 6 to 8 was used.
The catalytic rate constants \({\text{k}}_{{{\text{H}}^{ + } }}\) and \({\text{k}^{\prime}}_{{{\text{H}}^{ + } }}\) were calculated from the equation:
The kpH values from pH range 0.45 to 2.55 was used.
The plot \({\text{k}^{\prime}}_{\text{pH}} /{\text{a}}_{{{\text{H}}^{ + } }}\) = f(f2) is linear (Fig. 8). The catalytic rate constants \({\text{k}}_{{{\text{H}}^{ + } }}\) (1.44 × 10−4 mol−1 L s−1) and \({\text{k}^{\prime}}_{{{\text{H}}^{ + } }}\) (2.37 × 10−2 mol−1 L s−1) are equal to the values of \({\text{k}^{\prime}}_{\text{pH}} /{\text{a}}_{{{\text{H}}^{ + } }}\) for f2 = 1 and for f2 = 0, respectively.
The calculated theoretical profile of log k = f(pH) and that obtained from the experimental results were nearly identical, indicating that the choice of the equation describing the total rate of CFQ degradation was correct (Fig. 4). The pH rate profile demonstrated that CFQ is the most stable at pH 3–4 where the hydrolysis of di- and monocations of CFQ catalyzed by hydrogen ions and spontaneous hydrolysis of zwitter anions of CFQ occurs.
Stability of cefquinome sulfate in solid state
The degradation of cefquinome sulfate in solid state involves the influence of dry air to stimulate degradation in commercial packaging and the impact of increased temperature at 76% RH as well as of increased relative air humidity at 343 K. The degradation of CFQ in dry air and increased relative air humidity was the first-order reaction depending on the substrate concentration and is described by the Eq. 1. During the degradation of CFQ in the solid state, its concentration decreased in the time intervals t0 → t∞ from (ct)max to c0.
The effect of temperature
The determined rate constants (kobs) were used to calculate the Arrhenius relationship in order to interpret the influence of the temperature on the reaction rate at RH 76.4 and 0% RH, described by the following equation:
Here ki is the observed rate constants of cefquinome sulfate degradation (s−1), A is the frequency coefficient, Ea is the activation energy (J mol−1), R is the universal gas constant (8.3144 J K−1 mol−1), and T is the temperature (K).
The straight-line relationships ln ki = f(1/T) were obtained for CFQ in the temperature range 333–363 K, at increased relative air humidity (76.4%), and in dry air in the range 363–393 K. The least squares method was used to calculate the slopes (a) and the frequency coefficient (ln A), which allowed the calculation of activation energy (Ea = − a × R), enthalpy (∆H≠) and entropy (∆S≠) at 298 K (Table 2).
The activation energy of the degradation of cefquinome sulfate at RH = 0 and 76.4% is significantly different (Ea at RH 0% = 133.1 [kJ mol−1], Ea at RH 76.4% = 62.3 [kJ mol−1]), which was confirmed by the parallelism test: tcal. = 4.12 > t0.05 = 2.78. Thus, the storage conditions do affect the stability of the tested compound. The energy of activation of the compound analyzed at 0% RH is higher than at 76.4% RH. The entropy of activation at RH = 0 and 76.4% is negative, which may suggest the bimolecular character of the reaction with a significant spatial limitation.
The effect of humidity
The influence of relative air humidity on the stability of CFQ is described by the equation:
The semilogarithmic plot of ln ki = f(RH%) is a straight line and the slope of this curve (a) characterizes the effect of air humidity on the stability of the compound at 343 K, whereas the value exp(b) = k0 denotes its stability of CFQ at 343 K and at 0% RH. From the Arrhenius equation value of the rate constant of the degradation of cefquinome sulfate at 343 K in relative air humidity of 0% was calculated.
The extrapolated value ln kT=343K, RH 0% was compared with the value k0 of the degradation rate constant at 343 K and RH 0%. Determined from the dependence of ln ki = f(RH%) value k0 = 8.81 × 10−8 s−1 at 343 K and determined from the ln ki = f(1/T) value k343K, RH 0% = 9.46 × 10−9 s−1 for CFQ differ significantly and confirm the different mechanism of the degradation reaction of CFQ in air with RH 0% and in air with high humidity, despite the fact that the kinetic mechanism of its degradation is the same. In the solid state, CFQ is more stable in a dry atmosphere, and humidity constitutes the factor that determines the velocity of the degradative reaction.
Conclusion
The HPLC method with the separation parameters presented in this paper is suitable for the determination of the kinetic parameters of cefquinome degradation in the aqueous solutions and in the solid state. In aqueous solutions, in the pH range 0.45–10.48, the degradation of cefquinome sulfate is a pseudo-first-order reaction. The dissociation constants of CFQ in aqueous solutions were established (pKa 2.75 corresponding to the carboxylic group, pKa 3.75 to the primary amine group of the thiazol ring and pKa 7.54 to the tertiary amine group of the quinoline ring) by potentiometric titration and used to determine fractions of CFQ molecules depending pH (di- and monoprotonated molecules, zwitter ions and monoanions).
The hydrolysis of the diprotonated and protonated molecules of CFQ catalyzed by hydrogen ions, spontaneous hydrolysis of zwitter ions and monoanions of CFQ under the influence of water and degradation of CFQ molecules catalyzed by hydroxide ions are the partial reactions of degradation. The catalytic effect was observed for the components of the phosphate (pH 1.54–2.95), acetic (pH 3.66–5.47) and borate (pH 7.52–10.48) buffers. The bigger catalytic effect was observed according to acetate ions (an order of magnitude greater than acetic acid). Smaller differences were observed according to the components of the phosphate and borate buffer. CFQ is the most stable in the pH range 3–4.
The mechanism of the degradation of CFQ in the solid state depends on the storage conditions and occurs according to a first-order reaction depending on the substrate concentration in the presence of high relative air humidity, and at RH 0%. The susceptibility of CFQ to degradation under the influence of stress factors (pH, temperature, buffer components concentration, relative air humidity) should be considered during storage conditions and preparation of the product for administration.
In summary, the critical factors that influence CFQ stability are: temperature and relative air humidity in solid state and hydrogen and hydroxide ions as well as the components of phosphate, acetate and borate buffers in aqueous solutions. Care of these factors should be taken into consideration during manufacture and storage. Based on our study CFQ should be stored in tightly closed containers, protected from moisture and high temperature.
References
Kariyama T, Karasawa T, Nakagawa S, Yamamoto E (2002) Antimicrobial susceptibility of major pathogens of orofacial odontogenic infections to 11 β-lactam antibiotics. Oral Microbiol Immunol 17:285–289
Martin SI, Kaye KM (2004) Beta-lactam antibiotics: newer formulations and newer agents. Infect Dis Clin N Am 18:603–619
Sader HS, Jones RN (1993) The fourth-generation cephalosporins: antimicrobial activity and spectrum definitions using cefpirome as an example. Antimicrob Newsl 9:9–16
Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ (1991) Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning/impact of tissue penetration on infection response. Antimicrob Agents Chemother 35:1947–1959
Kaye KS, Engemann JJ, Fraimow HS, Abrutyn E (2004) Pathogens resistant to antimicrobial agents: epidemiology, molecular mechanisms, and clinical management. Infect Dis Clin N Am 18:467–511
Limbert M, Isert D, Klesel N, Markus A, Seeger K, Seibert G, Schrinner E (1991) Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob Agents Chemother 35:14–19
Ahmad I, Hao H, Huang L, Sanders P, Wang X, Chen D, Tao Y, Xiuhua K, Li J, Dan W, Yuan Z (2015) Integration of PK/PD for dose optimization of cefquinome against Staphylococcus aureus causing septicemia in cattle. Front Microbiol. doi:10.3389/fmicb.2015.00588
Shpigel NY, Levin ID, Winkler M, Saran A, Ziv G, Böttner A (1997) Efficacy of cefquinome for treatment of cows with mastitis experimentally induced using Escherichia coli. J Dairy Sci 80:318–323
Zonca A, Gallo M, Locatelli C, Carli S, Moroni P, Villa R, Cagnardi P (2011) Cefquinome sulfate behavior after intramammary administration in healthy and infected cows. J Dairy Sci 94:3455–3461
Ehinger AM, Schmidt H, Kietzmann M (2006) Tissue distribution of cefquinome after intramammary and “systemic” administration in the isolated perfused bovine udder. Vet J 172:147–153
Swinkels JM, Lam TJGM, Green MJ, Bradley AJ (2013) Effect of extended cefquinome treatment on clinical persistence or recurrence of environmental clinical mastitis. Vet J 197:682–687
Dołhań A, Jelińska A, Bębenek M (2014) Stability of ceftiofur sodium and cefquinome sulphate in intravenous solutions. Sci World J. doi:10.1155/2014/583461
Shantier SW, Gadkariem EA (2014) Colorimetric determination of cefquinome sulphate in bulk and dosage from using ammonium molybdate. Amer J Appl Sci 11:202–206
Uney K, Altan F, Elmas M (2011) Development and validation of a high-performance liquid chromatography method for determination of cefquinome concentrations in sheep plasma and its application to pharmacokinetics studies. Antimicrob Agents Chemother 55:854–859
Suhren G, Knappstein K (2003) Detection of cefquinome in milk by liquid chromatography and screening methods. Anal Chim Acta 483:363–372
Maes A, Meyns T, Sustronck B, Maes D, De Backer P, Croubels S (2007) Determination of cefquinome in pig plasma and bronchoalveolar lavage fluid by high-performance liquid chromatography combined with electrospray ionization mass spectrometry. J Mass Spectrom 42:657–663
Shantier SW, Gadkariem EA, Adam MO, Mohamed MA (2013) Development of stability-indicating methods for cefquinome sulphate. Int J Biomed Sci 9:162–167
Gans P, Sabatini A, Vacca A (1996) Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43:1739–1753
Pawełczyk E, Hermann T (1982) The fundamentals of stability of drugs (in polish). PZWL, Warszawa
Dołhań A, Jelińska A, Manuszewska M (2014) Stability-indicating HPLC method for the determination of cefquinome sulfate. Acta Polon Pharm Drug Res 71:249–264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dołhań, A., Urbaniak, B., Manuszewska, M. et al. Critical parameters for the stability of cefquinome sulfate in aqueous solutions and solid phase. Reac Kinet Mech Cat 122, 715–728 (2017). https://doi.org/10.1007/s11144-017-1257-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1257-0