Abstract
This work is devoted to the decomposition of gaseous HCN by oxidation with air catalyzed by different nickel containing catalysts. The presence of metallic nickel does not cause the decomposition of cyanide at 400 °C, but NiO, electrochemical prepared nickel oxides Ni2O3·xH2O and (β,γ)-NiOOH exhibit distinctly high catalytic activity at this temperature. The effect of HCN decomposition for electrochemically prepared nickel oxides was over 90 %. Nickel deposited on activated carbon also demonstrated catalytic behavior during the destruction of HCN with an air mixture at the temperature 400 °C. Nickel oxide mixed with activated carbon showed a small increase of catalytic activity in the destruction of HCN in comparison with NiO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanides are discharged by various industries, particularly in metallurgical processes (extraction of gold and silver), plating and surface finishing. They are considered among the most dangerous compounds for the environment and their toxicity is mainly due to their aptitude to release free cyanides. Different processes are proposed and used for the removal of cyanides in solutions and slurries [1–12].
Hydrogen cyanide (HCN) is a very toxic gas (ca. 30 times more toxic than carbon monoxide) that already affects human beings at ppm level in air whereas 1 min of exposure to concentrations of ca. 300 ppm is lethal [13]. However, to my knowledge, there are only a few publications focused on the catalytic decomposition of HCN in the gas phase, as reviewed by Kröcher and Elsner [14]. Iron oxide, Al2O3 or Pt, Pd, Rh, Ag, V2O5, WO3, CuSO4/TiO2 each supported on TiO2 pellets [15] and Pt/Al2O3 [16] were studied as catalysts in this reaction.
The activated carbons, which are known as an excellent adsorbents, are widely used as catalysts and supports of catalysts [10, 12, 17–19], in the mining industry for precious metal recovery and in polishing processes to remove low level cyanide concentration. For cyanide treatment, activated carbon and activated carbon impregnated with some transition metals have showed their effectiveness as an adsorbent and catalyst for the heterogeneous oxidation of cyanides and other pollutants. Although studies concerning cyanide adsorption with activated carbon have already been performed, the oxidation of HCN in the gas phase by nickel, nickel oxide and nickel-impregnated activated carbon has not yet been investigated.
HCN is ubiquitously produced from the pyrolysis of plastic wastes and solid fuels containing some organic nitrogen. The studies of HCN oxidation are justified due to the method of thermal decomposition of industrial waste, including hazardous waste, which very often contains cyanides because they are applied increasingly. It is worth taking into account that galvanic waste is also utilized by admixturing to ceramic building materials [20–24]. For this reason, there is a danger that trace quantities of cyanides can be released with waste gas.
Nickel hydroxide can be one of the post-galvanic waste components. Therefore, it is important to explain if the addition of nickel hydroxide to ceramics along with other galvanic waste can perform the function of catalyst in the oxidation of HCN within the ceramic material. Because the burned ceramic material has an alkaline reaction and conditions are oxidizing in a furnace [24], these are conducive to the oxidation of both nickel and HCN.
This work is dedicated to the removal of HCN emissions by oxidation using nickel, nickel oxides and nickel-impregnated activated carbon and silica, with the aim of finding new and improved catalysts for this reaction.
Materials and methods
Preparation of catalysts
Nickel powder (Inco-Europe Ltd.) type 255 grain-size distribution of 2.2–2.8 μm, nickel foam (Nitech-Sprapec) thickness 1.6 mm, density 500 g/m3 and porosity over 95 % and NiO (Aldrich) d < 10 μm was used as catalyst.
The starting material acting as a carrier was activated carbon NORIT RO 0.8 Supra No 92-70937 and silica SYLOID 244 (specific surface 300–350 m2/g).
Activated carbon and silica was modified by the deposition of the catalyst on their surface. This was a three-stage process. In the first stage, NORIT was degreased in the solution of mersolan in an ultrasonic washer at a temperature 70–80 °C for 30 min, silica was degreased for 15 min. Then the materials were thoroughly washed and filtered. After that, in the second stage, carbon and silica were activated for 30 min in the mixture of PdCl2 and SnCl2 in hydrochloric acid solution in an ultrasonic shaker. Such prepared materials were washed, filtered and dried. Ambient drying was preliminarily performed at room temperature for 20 h and further drying was continued at 80 °C for the next 2 h. In the next stage, dried samples were covered by a layer of nickel. The nickel layer was deposited using a chemical method. This process involved the immersing of the obtained materials in chemical nickel bath. All samples were ultrasonically stirred for 30 min at 70–80 °C. Afterwards, they were washed, filtered and dried at room temperature for 20 h and further at 80 °C to constant mass. The amount of deposited nickel onto carbon was about 25 %. Silica prepared in this way consisted of about 30 % of nickel, but ultrasonic stirring caused significant size reduction and inhomogeneity of the silica carrier. For this reason, the second part of the silica was covered by nickel using mechanical stirring. As a result of this process, silica with 18 % of nickel was obtained.
Additionally, a sample containing NiO powder mixed with carbon NORIT (in a 1:1 ratio) was prepared as a catalyst.
The Ni2O3·xH2O and (β,γ)-NiOOH samples were prepared by the anodic oxidation of NiO and β-Ni(OH)2 following the procedure outlined by Skowroński [25] and Czerwiński [26].
Experimental setup for HCN destruction
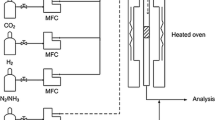
Catalytic HCN destruction measurements were carried out in a closed system consisting of three glass reactors, i.e. generation of HCN, oxidation and absorption reactors, a furnace NABERTHERM with programmable regulation of temperature and a water aspirator. The HCN generation reactor consisted of a distillation flask (1000 ml) with a dropping funnel, a capillary tube for bubbling air, and a reflux condenser with a pipe for carrying away the generated HCN. The oxidation reactor consisted of two parts. The bottom part included glassy foam. Catalysts were placed on this foam. The top part of the oxidation reactor possessed a pipe for carrying away gas products formed during HCN destruction to the absorption reactor, which consisted of three absorption bulbs with 1 % NaOH solutions (150 ml). The oxidation reactor was placed in the furnace.
Procedure of HCN destruction
The catalyst was placed in the reactor and in the furnace then NaCN solution (500 ml) containing 500 mg CN− was poured into the distillation flask. The water aspirator was started and the air was blown by the capillary tube which was immersed in NaCN solution. Then the furnace was turned on and after the proper temperature was reached, for 30 min (every 5 min), 10 ml of sulfuric acid solution (1:1) was poured through the dropping funnel. The total amount of added acid was 60 ml, and at this point, the distillation flask was heated up. The solution was boiled for 1 h. Next, the heating was turned off and the flask was cooled down for 0.5 h. Afterwards the capillary tube was removed and the system was aired for 0.5 h and then the furnace was turned off. The airflow was continued for another 0.5 h. Then the total content of cyanide in the absorption bulbs was determined.
Analytical procedure
The concentrations of cyanide in the reaction mixture were determined spectrophotometrically at λ max = 578 nm using pirydinebarbituric acid in the presence of Chloramine-T [27]. The UV-spectral analyses of cyanide were performed using a UV–VIS/MINI-1240 type photometer (SHIMADZU). All the chemicals used were analytical reagent grade.
Results and discussion
HCN oxidation by metallic nickel
The decomposition of HCN over nickel powder and nickel foam was investigated. These catalysts were placed in the oxidation reactor and the HCN/air mixture was let through. The data of these trials are reported in Table 1. It is shown that in a majority of cases, metallic nickel does not reveal catalytic behavior in the decomposition of cyanide. Only in one case of using nickel with a mass of 2.0087 g was the efficiency 14 %. In this case, one could observe that the powder glowed during the preliminary heating. It can mean that metallic nickel was partly oxidized to nickel oxide. The confirmation of this phenomenon is my subsequent studies with nickel oxide, which exhibited its catalytic behavior in the oxidation of HCN.
HCN oxidation by nickel oxides
In consecutive tests, NiO samples were placed in the catalytic vessel and HCN oxidation was studied at different temperatures (Table 2). At a temperature of 200 °C, the decomposition of HCN was not found, whereas at 300 and 350 °C, the decomposition ratio was significant. At the temperature 400 °C, this effect was over 90 %.
A similar relationship for electrochemically prepared Ni2O3·xH2O was observed (Table 3). Ni2O3·xH2O also exhibited distinctly high catalytic activity at this temperature.
In order to become convinced if in the process of HCN decomposing a nickel oxide sample loses its catalytic behavior, 9 trials with the same NiO weighed amount (2.0282 g) were carried out. Through this NiO sample, a total amount of 4.5 g HCN was let through (Fig. 1). As can be seen in Fig. 1, the catalytic activity of nickel oxide was not decreased in these trials. The decomposition of HCN was over 99 % in every test.
In the next series, the catalytic behavior of (β,γ)-NiOOH (as powder) was tested. In this case, only a smaller amount of electrode material was used (about 500 mg) in order to assure a low flow resistance through the layer of catalyst. The data shown in Table 3 prove unambiguously that electrochemically prepared (β,γ)-NiOOH has a high catalytic activity in the destruction of HCN. During further measurements (Fig. 1), the sample (β,γ)-NiOOH did not lose its catalytic activity.
HCN oxidation by nickel deposited on NORIT
The studies concern catalytic removal of cyanide ions in aqueous solutions using nickel catalyst—NiO exhibits its high catalytic activity at low temperatures [28] and investigations which had been carried out earlier into the decomposition of HCN in the gas phase confirmed its catalytic activity also in this case. Therefore, it was revealed purposely to develop a method of increasing NiO catalytic activity. With this aim, trials with a porous carrier coated in partly oxidized nickel were carried out. Activated carbon NORIT and silica SYLOID were the carriers.
First of all, the trials of HCN decomposition on carrier free nickel (NORIT and SYLOID) were carried out and their results are shown in Tables 4 and 5. It can be seen that HCN, without nickel catalyst, passed through the oxidation reactor with porous material present at the set, it did not decay and the decomposition is insignificant.
In connection with this, in the next trials in this reactor, a porous carrier with deposited nickel on it was placed. Because oxygen as well as HCN was also present in the stream flow and the decomposition process was carried out at relatively high temperatures (over 300 °C), an assumption that the formation of a nickel oxide layer took place on the nickel surface is justified. The data of trials with activated carbon/nickel catalyst are shown in Table 4. It can be seen that the presence of Ni/NiO on the carbon surface improves the rate of HCN removal significantly. At the temperature 300 °C, the efficiency of HCN decomposition was over 75 %. In the attempt at 350 °C the efficiency was over 91 %. Further studies at the temperature 400 °C continued with the same sample of Ni impregnated NORIT (Fig. 1) proved that the efficiency of HCN decomposition increases in comparison to the 0.5 g NiO sample and was over 80 % in the first two trials, but after subsequent trials, carbon in the reactor was burned.
The next studies were conducted using carbon NORIT mixed with NiO powder (in 1:1 ratio) and their results are shown in Fig. 1. The results demonstrate that the catalytic activity of NiO mixed with NORIT is higher than for a comparable sample of NiO without activated carbon but lower than for carbon covered with nickel using this chemical method.
HCN oxidation by nickel deposited on SYLOID
The results obtained for the second studied carrier silica SYLOID are presented in Table 5. In these cases, the layer of catalyst deposited in the reactor was higher than catalytic layers in previous studies. For this reason, sometimes disturbances of the process appeared affected, probably by the increase of HCN flow resistance through a thicker layer of catalysts. It can make gas penetration through the layer of catalyst difficult and it is possible that it was forming channels and as a result, the catalyst had a smaller contact with the faster flowing gas. Sometimes a flowing gas carries away silica particles. This phenomenon was especially visible for silica covered with 30 % of nickel. It was connected with its considerable size reduction during the ultrasonic stirring.
Taking into account that mixing cyanides with nickel ions is avoided due to the generation of a very stable complex (the stability product of Ni(CN) 2−4 is 1031,3), the next trials examined if the observed loss of HCN was connected to its bonding with nickel. With this end in view, the samples of catalysts were investigated for cyanide content. In these studies, the samples were placed into a distillation flask and the whole procedure of cyanide absorption was conducted, except that there was no catalyst in the oxidation reactor and the furnace was not turned on. If the samples of catalyst contained cyanide bonding with nickel, the emission of HCN after the addition of concentrated sulfurous acid (1:1) would be unavoidable. But the presence of cyanide was not found in any samples.
Conclusion
Metallic nickel does not reveal catalytic behavior in the decomposition of cyanide with an air mixture at 400 °C, but NiO and electrochemically prepared nickel oxides Ni2O3·xH2O and (β,γ)-NiOOH exhibit distinctly high catalytic activity at this temperature. The amount of HCN decomposition with electrochemically prepared nickel oxides was over 90 %. It is a reasonable assumption that Ni(III) ion present at the oxide surface take part in HCN heterogenous destruction and their catalytic activity depends on both their superficial concentration and reaction temperature.
Nickel deposited on activated carbon also demonstrates catalytic behavior during the destruction of HCN with air mixture at the temperature 400 °C. But activated carbon is burned during the measurements.
NiO mixed with activated carbon in the destruction of HCN showed a small increase of catalytic activity in comparison with NiO.
References
Mihaylov BV, Hendrix JL (1993) JH Nelson. J Photochem Photobiol A: Chem 72:173–177
Hidaka H, Nakamura T, Ishizaka A, Tsuchiya M, Zhao J (1992) J Photochem Photobiol A: Chem 66:367–374
Augugliaro V, Loddo V, Marcì G, Palmisano L, López-Muñoz MJ (1997) J Catal 166:272–283
Rose TL, Nanjundiah C (1985) J Phys Chem 89:3766–3771
Peral J, Muñoz J, Domènech X (1990) J Photochem Photobiol A: Chem 55:251–257
Kogo K, Yoneyama H, Tamura H (1980) J Phys Chem 84:1705–1710
Serpone N, Borgarello E, Barbeni M, Pelizzetti E, Pichat P, Hermann JM, Fox MA (1987) J Photochem 36:373–388
Ahmed MS, Attia YA (1995) J Non-Cryst Solid 186:402–407
Chiang K, Amal R, Tran T (2003) J Mol Cat A: Chem 193:285–297
Yeddou AR, Chergui S, Chergui A, Halet F, Hamza A, Nadjemi B, Ould-Dris A, Belkouch J (2011) Miner Eng 24:788–793
Yazici EY, Deveci H, Alp I (2009) J Hazard Mater 166:1362–1366
Yeddou AR, Nadjemi B, Halet F, Ould-Dris A, Capart R (2010) Miner Eng 23:32–39
Luxon SG (1992) Hazards in the chemical laboratory. Royal Society of Chemistry, Cambridge
Kröcher O, Elsner M (2009) Appl Catal B 92:75–89
Miyadera T (1998) Appl Catal B 16:155–164
Zhao H, Tonkyn RG, Barlow SE, Koel BE, Peden CHF (2006) Appl Catal B 65:282–290
Faria PCC, Orfao JJM, Pereira MFR (2005) Water Res 3/8:1461–1470
Mackenzie K, Battke J, Kopinke FD (2005) Catal Today 102–103:148–153
Adhoum N, Monser L (2002) Chem Eng Process 41/1:17–21
Budilovsky J (1991) The Economist of Lithuania 3:61–64
Osińska M, Stefanowicz T, Paukszta D (2003) Waste Manag 23:871–877
Stefanowicz T, Osińska M, Nowak M (2003) Polish J App Chem 47:23–30
Osińska M (2001) Stefanowicz. Polish J App Chem 45:109–116
Stefanowicz T, Słowik M, Osińska M (2001) Chemik LIV/5:111–113
Skowroński JM, Ważny A (2006) J New Mat Electr Sys 9:345–351
Czerwiński A, Dmochowska M, Grdeń M, Kopczyk M, Wójcik G, Młynarek G, Kołata J, Skowroński JM (1999) J Pow Sour 77:28–33
Polish Standard, PN-80. C-04603.01, Water and waste water. Test for cyanides
Christoskova St G, Stojanova M, Georgieva M (1999) React Kinet Catal Lett 67:59–66
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Osińska, M. Decomposition of gaseous HCN in the presence of Ni-containing catalysts. Reac Kinet Mech Cat 109, 57–65 (2013). https://doi.org/10.1007/s11144-012-0535-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0535-0