Abstract
The influence of pH on the stability of cefoselis sulfate was investigated in the pH range 0.44–13.00. The degradation of cefoselis sulfate in aqueous solutions was a pseudo-first order reaction. General acid–base hydrolysis of cefoselis sulfate was observed in phosphate and acetate buffers. In solutions of hydrochloric acid, sodium hydroxide and borate buffer, kobs = kpH because only specific acid–base catalysis occurred. Specific acid–base catalysis of cefoselis sulfate involved such reactions as the hydrolysis of protonated cefoselis sulfate molecules (k1), hydrolysis of cefoselis sulfate zwitter ions (k2) and hydrolysis of cefoselis sulfate monoanions (k3) under the influence of water. The total reaction rate was equal to the sum of partial reactions: kpH = k1·F1 + k2·F2 + k3·F3 where: k1, k2 and k3 were the catalytic rate constants calculated as the mean values of kpH at pH 1.89–3.10, 4.01–6.16 and at pH above 11.24. F1, F2 and F3 were the molar concentration fractions of the cefoselis sulfate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The class of cephalosporins came into being in 1954. Since the isolation of the first cephalosporin, they have been increasingly applied in the treatment of various types of infections. At the moment, there are over twenty cephalosporin antibiotics available commercially.
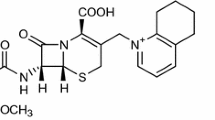
As the antibacterial activity of cephalosporins is connected with their resistance to alkaline hydrolysis, their therapeutic effect depends on the stability of the β-lactam moiety, which is often related to the substituent at position three [1–3] (Fig. 1).
In a molecule of cephalosporin, the constituent that is the most prone to degradation is the β-lactam moiety. Any structural modifications aimed at greater alkaline stability usually result in enhanced vulnerability to degradation in an acidic environment. In principle, antibiotics are expected to be stable at physiological and acidic pH, especially when designed for oral administration [1–3]. With a view to create acid-stable antibiotics, cephalosporins are currently the main target of research into new anti-infection drugs [1]. It has been found that cephalosporins are vulnerable to degradation in aqueous solutions [4–8] and in the solid state [9–15].
Cefoselis sulfate (Fig. 1) is a novel, parenteral, fourth-generation cephalosporin. It has a broad spectrum of antibacterial activity against Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa [16–18]. Cefoselis sulfate contains a 3-non-acetoxy group at position C3, which determines its antibacterial activity against methicillin-resistant Staphylococcus aureus. This group is probably responsible for a greater stability of cephalosporins in acidic than in alkaline media and a wide plateau region at neutral pH [1].
The aim of this work was to evaluate the stability of cefoselis sulfate at a wide pH range and to determine the pH range at which it is the most stable. An HPLC method described previously was used to determine acid–base catalysis of cefoselis sulfate [19].
Experimental procedure
Chemicals, reagents, and solutions
Cefoselis sulfate was obtained from Xingcheng Chempharm Co., Ltd. Taizhou, Zhejiang, China. It is a white or light yellow crystalline powder containing 99.5 % cefoselis sulfate, 0.1 % related substances and complies with the Chinese Pharmacopoeia 2005 regulations.
All other chemicals and solvents were obtained from Merck KGaA (Germany) and were of analytical grade. High quality pure water was prepared by using the Millipore purification system (Millipore, Molsheim, France, model Exil SA 67120).
Instrumentation
Chromatographic separation and quantitative determination of cefoselis sulfate were conducted by using a high-performance liquid chromatograph equipped with an LC-6A pump (Shimadzu), a UV–Vis (SPD-6AV) detector (Shimadzu) and a Rheodyne with a 50 μL loop. As the stationary phase, a LiChrospher RP-18 column, 5 μm particle size, 250 × 4 mm (Merck, Darmstadt, Germany) was used. The mobile phase consisted of 5 volumes of acetonitrile and 95 volumes of ammonium acetate, 12 mmol L−1, the pH of the mobile phase was 7.15. The flow rate of the mobile phase was 1.0 mL min−1. The wavelength of the detector was set at 260 nm. The HPLC method has been evaluated and validated for the determination of cefoselis sulfate in stability studies [19].
Kinetic studies
The degradation of cefoselis sulfate in aqueous solutions was examined at 353 K in hydrochloric acid (pH 0.44–1.39), phosphate buffer (pH 1.89–3.10 and 6.16–7.60), acetate buffer (pH 4.01–5.65) and borate buffer (pH 8.01–9.67). The degradation of cefoselis sulfate in aqueous solutions of sodium hydroxide (pH 11.24–13.00) was examined at 298, 303, 308 and 313 K and the obtained results were extrapolated to 353 K. The pH values of the reaction solutions and those of the buffer used to calibrate the pH-meter were measured at reaction temperature. The pH values of the reaction solutions in HCl were calculated from the equation pH = −log·aHCl [HCl]. The activity coefficients aHCl were obtained from the literature or calculated by interpolation of literature data [20]. The buffers were prepared according to European Pharmacopoeia 7th Edition. The pH values of buffers were calculated using average pH value of different concentration buffers. The ionic strength of solutions was adjusted to 0.5 mol L−1 using the solution of sodium chloride (4 mol L−1). All solutions of cefoselis sulfate were protected from light. Degradation was started by dissolving an accurately weighed sample of cefoselis sulfate (2.5 mg) in 12.5 mL of reaction solution heated to the required temperature in stoppered flasks. At specified time intervals, which were determined by the rate of degradation, samples of the reaction solutions (0.5 mL) were collected. Then they were neutralized if necessary and instantly cooled in the mixture of ice and water. 50 μL samples of the solutions were injected into the column.
Results and discussion
The HPLC method with UV detection used in this study was found suitable for the determination of cefoselis sulfate under the stress conditions of hydrolysis (acid and base), oxidation, photolysis and thermal degradation [19]. The selectivity of this method for the determination of cefoselis sulfate in the presence of degradation products was confirmed. The symmetrical peak of cefoselis sulfate (tR = 14.58 min) was clearly separated from the peaks of the degradation products formed in the whole pH range (Fig. 2).
The degradation of cefoselis sulfate was a pseudo-first order reaction described by the following equation:
where ct and c0 are the time-dependent concentration and the initial concentration of cefoselis sulfate, at time t > 0 and t = 0, respectively, kobs is the observed rate constant of cefoselis sulfate degradation. The semi-logarithmic plots ln ct = f(t) (Fig. 3) were linear and their slopes were equal to the rate constants of the reactions with the negative sign (−kobs). The number of measurements of ct for each series ranged from 8 to 12. The observed rate constants of cefoselis sulfate were determined in the pH range 0.44–13.00.
Buffer catalysis
The fact that at a constant pH, ionic strength and temperature, in the presence of buffer excess, the observed rate constants depended on the total concentration of buffer demonstrated only general acid–base catalysis occurred, which was observed in the case of the acetate (pH 4.01–5.65) and phosphate (pH 1.89–3.10 and 6.16–7.61) buffers. The first order rate constants kpH under the conditions of general acid–base catalysis were calculated from the following equation:
where kpH is the rate constant at zero buffer concentration, kB is the catalytic effect of the buffer, [BT] is the total buffer concentration.
The plots kobs = f([BT]) obtained for the acetate (Fig. 4) and phosphate (Fig. 5) buffers were linear and their slopes equaled kB whereas the values of kobs for [BT] = 0 equaled kpH.
The catalytic effect of the acetate buffer was investigated at pH 4.01–5.65. The observed rate constants were calculated from the equation:
where AcH is the undissociated acetic acid and Ac is the acetate ion. The total acetate concentration BT, is:
The observed rate constant was calculated from the pKa of the acetic acid (4.52) and Eqs. 2 and 3.
From the slopes of the plots, kobs = f(BT) for each pH, the buffer catalytic rate constants were calculated. The buffer catalytic effect of the phosphate buffer (pH 1.89–3.10, pKa = 2.10 and pH 6.16–7.61, pKa = 6.58) was calculated thus:
From Eqs. 6, 7 kpH and the buffer catalytic rate constants of the acetate and phosphate buffer components were calculated (Table 1).
In the reaction solutions in HCl, borate buffer and NaOH, general acid–base catalysis was not observed, therefore the values of kobs were equal to kpH. To verify that the differences between kobs values determined at different buffer concentrations were statistically insignificant, the parallelism test was used.
pH-rate profile
The values of kpH determined in hydrochloric acid, sodium hydroxide, phosphate, borate and acetate buffers were used to calculate the relationship log kpH = f(pH) (Fig. 6). The semilogarithmic relationship kpH-pH indicates that in aqueous solutions (pH 0.44–13.00) the following reactions are possible:
-
degradation of a protonated cefoselis sulfate molecule (k1)
-
degradation of cefoselis sulfate zwitter ions (k2)
-
degradation of cefoselis sulfate monoanions (k3)
under the influence of water.
Log kpH = f(pH) profile for the degradation of cefoselis sulfate at 353 K. The points are determined experimentally and the lines were calculated from Eq. 8
The total reaction rate is equal to the sum of partial reaction rates:
where F1, F2 F3—the molar concentration fractions of cefoselis sulfate. The values F1–3 were calculated from the pKa values of cefoselis sulfate (2.8 and 10.4). The catalytic rate constants k1–3 were calculated as the mean values of kpH at pH 1.89–3.10, 4.01–6.16 and at pH above 11.24.
The correctness of Eq. 8 was verified by a good agreement between the theoretical profile calculated from Eq. 8 and the experimental results (Fig. 6).
The influence of ionic strength
The ionic strength in hydrochloric acid and in sodium hydroxide solution did not affect the stability of cefoselis sulfate, which confirmed that only spontaneous hydrolysis of cefoselis sulfate took place.
Conclusion
In aqueous solutions, in the pH range 0.44–13.00, the degradation of cefoselis sulfate is a pseudo-first order reaction. The hydrolysis of the protonated molecules, zwitter ions and monoanions of cefoselis sulfate constitutes the total reaction of hydrolysis. A catalytic effect was observed in the phosphate (pH 1.89–3.10 and 6.16–7.61) and acetate (pH 4.01–5.65) buffers. Cefoselis sulfate was the most stable at pH 4.0–6.5 and the least stable above pH 11.24.
The relationship log kpH = f(pH) of cefoselis sulfate indicates that it is more stable in acidic than in alkaline media and that it is characterized by a wide plateau region at acidic and neutral pH, which confirms the influence of the 3-non-acetoxy group at position 3 (Fig. 1) on the stability of this cephalosporin.
The catalytic effect of phosphate and acetate buffer components on the degradation of cefoselis sulfate is significant and does not depend on the buffer type, whereas the components of a borate buffer do not catalyze the degradation of cefoselis sulfate. The stability of cefoselis sulfate in the presence of a borate buffer is lower than when phosphate or acetate buffers are used, despite their catalytic effect. Therefore, the components of acetate, borate and phosphate buffers are to be avoided as excipients in pharmaceutical formulations of cefoselis sulfate.
References
Akaho E, Nakayama H (2003) J Antibiot (Tokyo) 56(4):379–391
Flessner T, Jautelat R, Scholz U, Winterfeldt E (2004) Fortschr Chem Org Naturst 87:1–80
Hwu JR, Ethiraj KS, Hakimelahi GH (2003) Mini Rev Med Chem 3(4):305–313
Patel G, Rajput S (2011) Acta Chromatogr 23(2):215–234
Ikeda Y, Ban J, Ishikawa T, Hashiguchi S, Urayama S, Horibe H (2008) Chem Pharm Bull 56(10):1406–1411
Jelińska A, Dobrowolski L, Oszczapowicz I (2004) J Pharm Biomed Anal 35(5):1273–1277
Sugioka T, Asano T, Chikaraishi Y, Suzuki E, Sano A, Kuriki T, Shirotsuka M, Saito K (1990) Chem Pharm Bull 38(7):1998–2002
Fubara JO, Notari RE (1998) J Pharm Sci 87(12):1572–1576
Medenecka B, Jelińska A, Zając M, Bałdyka M, Juszkiewicz K, Oszczapowicz II (2009) Acta Pol Pharm 66(5):563–569
Jelińska A, Medenecka B, Zając M, Knajsiak M (2008) Acta Pol Pharm 65(2):261–265
Zając M, Jelińska A, Zalewski P (2005) Acta Pol Pharm 62(2):89–94
Jelińska A, Dudzińska I, Zając M, Oszczapowicz I, Krzewski W (2005) Acta Pol Pharm 62(3):183–187
Zając M, Jelińska A, Dobrowolski L, Oszczapowicz I (2003) J Pharm Biomed Anal 32(6):1181–1187
Jelińska A, Zając M, Gostomska J, Szczepaniak M (2003) Farmaco 58(4):309–313
Jelińska A, Zając M, Jakubowska M (2001) React Kinet Catal Lett 73(2):325–331
Kuriyama T, Karasawa T, Nakagawa K, Nakamura S, Yamamoto E (2002) Oral Microbiol Immunol 17(5):285–289
Climo MW, Markowitz SM, Williams DS, Hale-Cooper CG, Archer GL (1997) J Antimicrob Chemother 40(1):59–66
Zalewski P, Cielecka-Piontek J (2011) Ann Acad Med Siles 65(3):77–81
Zalewski P, Cielecka-Piontek J, Jelińska A (2012) Cent Eur J Chem 10(1):121–126
Pawełczyk E, Hermann T (1982) The fundamentals of stability drugs (Poland). PZWL, Warsaw
Acknowledgments
This study was supported by a Grant from the State Committee for Scientific Research, Poland (No. NN405 683040).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zalewski, P., Cielecka-Piontek, J. & Jelińska, A. Stability of cefoselis sulfate in aqueous solutions. Reac Kinet Mech Cat 108, 285–292 (2013). https://doi.org/10.1007/s11144-012-0523-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0523-4