Abstract
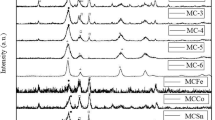
A series of Mn–Ce(M) solids (M = K or Na), with molar ratios 100–0, 50–50 and 0–100 were prepared by co-precipitation of manganese and cerium nitrate from NaOH or KOH solutions at pH = 11. In addition, part of the solids precipitated with NaOH were dried and impregnated with a Cu2+ salt. The solids were characterized by XRD, Specific Surface Area, XPS and EDS. The characterization analyses show the formation of Mn mixed oxides with different oxidation states (Mn3+, Mn4+), for samples without Ce or Mn–Ce(M) 50–50. In the latter solid and in the one where there is no Mn, the formation of CeO2 (fluorite type) was detected. The samples were tested in the phenol removal in water at 100 °C and at atmospheric pressure with the aim to analyze the adsorbed species in the first stage of the adsorption-oxidation mechanisms. The results indicate, on the one hand, that [MnOx] is the active species in the process and that the most active solids are those that present (i) a higher concentration of OI, (ii) a higher amount of Mn4+ ions. DRIFT spectroscopy showed a possible mechanism of phenol adsorption on two sites, in the first one by H interaction of OH (phenol) with an OH of the catalyst and in the second, by the formation of a phenolate species between an O (OH phenol) and Mnn+.









Similar content being viewed by others
References
Jordan W, van Barnevel H, Gerlich O, Kleine M, Ulrico J (2002) “Ullmann’s encyclopaedia of industrial” chemistry. Wiley-VCH Verlag, New York
Canadian Environmental Protection Act, Priority Substances List Assessment Report: Phenol (2000) Minister of Public Works and Government Services
Busca G, Berardinelli S, Resini C, Arrighi L (2008) J Hazard Mater 160:265–288
Sanabria N, Molina R, Moreno S (2010) Catal Lett 130:664–671
Resini C, Catania F, Berardinelli S, Paladino O, Busca G (2008) Appl Catal B 84:678–683
Zhar S, Wang X, Huo M (2010) Appl Catal B 97:127–134
Hu B, Chen C, Frueh S, Jin L, Joesten R, Suib S (2010) J Phys Chem 114:9835–9844
Abecassis-Wolfovich M, Landau M, Brenner A, Herskowitz M (2004) Ind Eng Chem Res 43:5089–5097
Arena F, Negro J, Parmaliana A, Spadaro L, Trunfio G (2007) Ind Eng Chem Res 46:6724–6731
Kouraichi R, Delgado J, López Castro J, Stitou M, Rodriguez Izquierdo J, Cauqui M (2010) Catal Today 154:195–201
Imamura S, Dol A, Ishida S (1985) Ind Eng Chem Prod Res Dev 24:75–80
Tang X, Chen J, Li Y, Li Y, Xu Y, Shen W (2006) Chem Eng J 118:119–125
Hussain S, Sayari A, Larachi F (2001) Appl Catal B 34:1–9
Larachi F, Pierre J, Adnot A, Bernis A (2002) Appl Surf Sci 195:236–250
Xing X, Yu P, Xu M, Wu X, Li S (2008) J Phys Chem C 112:15526–15531
Galakhov V, Demeter M, Bartkowski S, Neumann M, Ovechkina N, Kurmaev E, Lobachevskaya N, Ya M, Mukovskii J, Mitchell J, Ederer D (2002) Phys Rev B 65:113102–113106
Oku M (1995) J Elec Spectrosc Relat Phenom 74:135–148
Tang X, Li Y, Huang X, Xu Y, Zhu H, Wang J, Shen W (2006) Appl Catal B 62:265–273
Chapelle A, Yaacob M, Pasquet I, Presmanes L, Bernabé A, Tailhades P, Du Pleiss J, Kalantarzadeh K (2011) Sens Actuators B: Chem 153:117–124
Ji P, Zhang J, Chen F, Anpo M (2008) J Phys Chem C 112:17809–17813
Damynova S, Bueno J (2003) Appl Catal A 253:135–141
Abecassis-Wolfovich M, Jothiramalingam R, Landau M, Herskowitz M, Viswanathan B, Varadarajan T (2005) Appl Catal B 59:91–98
Rives V, Del Arco M, Prieto M (2004) Bol Soc Esp Ceram V 43:142–147
Peluso M, Sambeth J, Thomas H (2003) React Kinect Catal Lett 80:41–47
Li T, Chiang S, Liaw B, Chen Y (2011) Appl Catal B 103:143–148
Li J, Zhu P, Zhou R (2011) J Power Sour 196:9590–9598
Cheng D, Hou Ch, Chen F, Zhan X (2009) React Kinet Catal Lett 97:217–223
Magne P, Walker P (1986) Carbon 24:101–107
Santiago A, Sousa J, Guedes R, Jerônimo C, Benachour M (2006) J Hazard Mater 138:325–330
Cheng D, Hou Ch, Chen F, Zhan X (2009) J Rare Earths 27:723–727
Arena F, Italian C, Ranieri A, Saja C (2010) Appl Catal B 99:321–328
Bride M, Kung K (1991) Environ Toxicol Chem 10:441–448
Bhargava S, Tardio J, Prasad J, Folger K, Akolekar D, Grocott S (2006) Ind Eng Chem Res 45:1221–1258
Hamoudi S, Sayari A, Belkacemi K, Bonneviot L, Larachi F (2000) Catal Today 62:379–388
Zhang H, Yang W, Li D, Wang X (2009) React Kinect Catal Lett 97:263–268
Picasso G, Gutiérrez M, Pina M, Herguido J (2007) Chem Eng J 126:119–130
Shi L, Chu W, Qu F, Luo S (2007) Catal Lett 113:59–64
Mariey L, Lamotte J, Lavalley J, Tsyganenko N, Tsyganenko A (1996) Catal Lett 41:209–211
Abbas O, Rebufa C, Dupuy N, Kister J (2008) Talanta 77:200–209
Tang X, Chen J, Li Y, Li Y, Xu Y, Shen W (2006) Chem Eng J 118:119–125
Lamaita L, Peluso M, Thomas H, Sambeth J, Minelli G, Porta P (2005) Catal Today 107–108:133–138
Sambeth J, Juan A, Gambaro L, Thomas H (1997) J Mol Catal A: Chem 118:283–291
Andrade L, Laurindo E, de Oliveira R, Rocha-Filho R, Cass Q (2006) J Braz Chem Soc 17:369–373
Acknowledgments
The authors are grateful to CONICET, UNLP and ANPCYT of Argentina for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’alessandro, O., Thomas, H.J. & Sambeth, J.E. An analysis of the first steps of phenol adsorption-oxidation over coprecipitated Mn–Ce catalysts: a DRIFTS study. Reac Kinet Mech Cat 107, 295–309 (2012). https://doi.org/10.1007/s11144-012-0470-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0470-0