Abstract
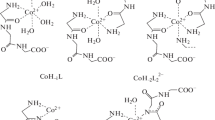
Carboxylate anions in aqueous solutions coordinate with the Tc(CO) +3 ion mainly in the monodentate fashion to form weak 1 : 1, 1 : 2, and 1 : 3 complexes. The stability of the complexes decreases with increasing size of the alkyl radical. In the series of halo-substituted acetates, all the stability constants decrease with a decrease in the basicity of the anion.
Similar content being viewed by others
REFERENCES
Schwochau, K., Technetium, Weinheim: Wiley, 2000.
Alberto, R., Schibli, R., and Waibel, R., et al., Coord. Chem. Rev., 1999, vols. 190–192, pp. 901–919.
Lumpov, A.A., Gorshkov, N.I., Miroslavov, A.E., et al., Radiokhimiya, 2000, vol. 42, no.3, pp. 231–235.
Lumpov, A.A., Gorshkov, N.I., Miroslavov, A.E., et al., Radiokhimiya, 2003, vol. 45, no.2, pp. 116–119.
Alberto, R., Schibli, R., Angst, D., et al., Transition Met. Chem., 1997, vol. 22, pp. 596–601.
Author information
Authors and Affiliations
Additional information
__________
Translated from Radiokhimiya, Vol. 47, No. 1, 2005, pp. 49–52.
Original Russian Text Copyright © 2005 by Suglobov, Miroslavov, Sidorenko, Gorshkov, Lumpov.
Rights and permissions
About this article
Cite this article
Suglobov, D.N., Miroslavov, A.E., Sidorenko, G.V. et al. Complexation of Tc(CO) +3 ·aq with Anions of Monobasic Carboxylic Acids in Aqueous Solutions: A 99Tc NMR Study. Radiochemistry 47, 50–53 (2005). https://doi.org/10.1007/s11137-005-0046-1
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11137-005-0046-1