Abstract
Purpose
To examine whether a 7-day or 24-h recall period of Perioperative Symptom Assessment for Patients Undergoing Lung Surgery (PSA-Lung) was appropriate for symptom assessment after discharge.
Methods
A total of 377 patients were recruited in a cohort study of patients who underwent lung surgery. We measured patient symptoms daily and weekly using the two recall period versions of the PSA-Lung scale, respectively. The psychometric properties of both versions were calculated. Spearman rank correlation coefficients and kappa (k) coefficients were used to measure the association between items score measured by the two version scales each week. Cohen’s d effect size and mixed linear model were used to measure responsiveness to change over time.
Results
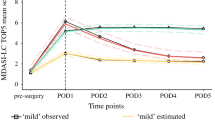
Spearman rank correlation coefficients between the symptom scores generated by the 7-day and 24-h versions (range 0.48–0.77; all P < 0.05). The correlations increased in patients in stable condition (weekly symptom change < 2). Cronbach’s α coefficients for both ratings were > 0.87 and both had good test–retest reliability. The longitudinal analysis and Cohen’s d effect sizes showed that both ratings had good ability to detect changes in all items.
Conclusion
The 7-day retrospective scale was as effective as the 24-h retrospective scale in terms of psychometric performance. In the stage where the patient’s symptoms change rapidly, it is recommended to use the 24-h retrospective scale for symptom monitoring. On the contrary, in a stable state, it can be considered to use the 7-day retrospective scale for monitoring to reduce the patient’s burden.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author [Qiuling Shi].
References
de Koning, H. J., van der Aalst, C. M., de Jong, P. A., Scholten, E. T., Nackaerts, K., Heuvelmans, M. A., Lammers, J. J., Weenink, C., Yousaf-Khan, U., Horeweg, N., et al. (2020). Reduced lung-cancer mortality with volume CT screening in a randomized trial. New England Journal of Medicine, 382(6), 503–513.
Fagundes, C. P., Shi, Q., Vaporciyan, A. A., Rice, D. C., Popat, K. U., Cleeland, C. S., & Wang, X. S. (2015). Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. Journal of Thoracic and Cardiovascular Surgery, 150(3), 613-619.e612.
Ljungqvist, O., Scott, M., & Fearon, K. C. (2017). Enhanced recovery after surgery: A review. JAMA Surgery, 152(3), 292–298.
Huang, L., Frandsen, M. N., Kehlet, H., & Petersen, R. H. (2022). Early and late readmissions after enhanced recovery thoracoscopic lobectomy. European Journal of Cardiothoracic Surgery, 62, 3.
Bao, F., Dimitrovska, N. T., Hu, S., Chu, X., & Li, W. (2020). Safety of early discharge with a chest tube after pulmonary segmentectomy. European Journal of Cardio-Thoracic Surgery, 58(3), 613–618.
Vandrevala, T., Senior, V., Spring, L., Kelliher, L., & Jones, C. (2016). “Am I really ready to go home?”: A qualitative study of patients’ experience of early discharge following an enhanced recovery programme for liver resection surgery. Supportive Care in Cancer, 24(8), 3447–3454.
Zhang, J., Su, X., Xu, W., Yu, Q., Dai, W., Wang, Y., Zhuang, X., Li, Q., Wang, X. S., & Shi, Q. (2022). Identifying patients who suffered from post-discharge cough after lung cancer surgery. Supportive Care in Cancer, 30(9), 7705–7713.
Wang, K. Y., Chang, N. W., Wu, T. H., Hsu, C. C., Lee, Y. H., & Lee, S. C. (2010). Post-discharge health care needs of patients after lung cancer resection. Journal of Clinical Nursing, 19(17–18), 2471–2480.
Lowery, A. E., Krebs, P., Coups, E. J., Feinstein, M. B., Burkhalter, J. E., Park, B. J., & Ostroff, J. S. (2014). Impact of symptom burden in post-surgical non-small cell lung cancer survivors. Supportive Care in Cancer, 22(1), 173–180.
Dai, W., Feng, W., Zhang, Y., Wang, X. S., Liu, Y., Pompili, C., Xu, W., Xie, S., Wang, Y., Liao, J., Wei, X., Xiang, R., Hu, B., Tian, B., Yang, X., Wang, X., Xiao, P., Lai, Q., Wang, X., … Shi, Q. (2022). Patient-reported outcome-based symptom management versus usual care after lung cancer surgery: A multicenter randomized controlled trial. Journal of Clinical Oncology, 40(9), 988–996.
Guidance for industry: patient-reported outcome measures: Use in medical product development to support labeling claims: draft guidance. (2006). Health and Quality of Life Outcomes, 4, 79.
de Bono, J., Mateo, J., Fizazi, K., Saad, F., Shore, N., Sandhu, S., Chi, K. N., Sartor, O., Agarwal, N., Olmos, D., Thiery-Vuillemin, A., Twardowski, P., Mehra, N., Goessl, C., Kang, J., Burgents, J., Wu, W., Kohlmann, A., Adelman, C. A., & Hussain, M. (2020). Olaparib for metastatic castration-resistant prostate cancer. New England Journal of Medicine, 382(22), 2091–2102.
Kallen, M. A., Yang, D., & Haas, N. (2012). A technical solution to improving palliative and hospice care. Supportive Care in Cancer, 20(1), 167–174.
Bergman, B., Aaronson, N. K., Ahmedzai, S., Kaasa, S., & Sullivan, M. (1994). The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. European Journal of Cancer, 30(5), 635–642.
Cella, D. F., Bonomi, A. E., Lloyd, S. R., Tulsky, D. S., Kaplan, E., & Bonomi, P. (1995). Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer, 12(3), 199–220.
Mendoza, T. R., Wang, X. S., Lu, C., Palos, G. R., Liao, Z., Mobley, G. M., Kapoor, S., & Cleeland, C. S. (2011). Measuring the symptom burden of lung cancer: The validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. The Oncologist, 16(2), 217–227.
Hollen, P. J., Gralla, R. J., Kris, M. G., & Potanovich, L. M. (1993). Quality of life assessment in individuals with lung cancer: Testing the Lung Cancer Symptom Scale (LCSS). European Journal of Cancer, 29A(Suppl 1), S51-58.
Dalziel, K., Li, J., Scott, A., & Clarke, P. (2018). Accuracy of patient recall for self-reported doctor visits: Is shorter recall better? Health Economics, 27(11), 1684–1698.
Guidance for industry: Principles for Selecting, Developing, Modifying, and Adapting Patient-Reported Outcome Instruments for Use in Medical Device Evaluation.[8]Food and Drug Administration.pdf. (2020).
Rolstad, S., Adler, J., & Rydén, A. (2011). Response burden and questionnaire length: Is shorter better? A review and meta-analysis. Value Health, 14(8), 1101–1108.
Gabrilove, J. L., Perez, E. A., Tomita, D. K., Rossi, G., & Cleeland, C. S. (2007). Assessing symptom burden using the M.D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer, 110(7), 1629–1640.
Northouse, L. L., Mood, D. W., Schafenacker, A., Montie, J. E., Sandler, H. M., Forman, J. D., Hussain, M., Pienta, K. J., Smith, D. C., & Kershaw, T. (2007). Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer, 110(12), 2809–2818.
Bennett, A. V., Patrick, D. L., Bushnell, D. M., Chiou, C. F., & Diehr, P. (2011). Comparison of 7-day and repeated 24-h recall of type 2 diabetes. Quality of Life Research, 20(5), 769–777.
Schaffer, E. M., Basch, E. M., Schwab, G. M., & Bennett, A. V. (2021). Comparison of weekly and daily recall of pain as an endpoint in a randomized phase 3 trial of cabozantinib for metastatic castration-resistant prostate cancer. Clinical Trials, 18(4), 408–416.
Shi, Q., Trask, P. C., Wang, X. S., Mendoza, T. R., Apraku, W. A., Malekifar, M., & Cleeland, C. S. (2010). Does recall period have an effect on cancer patients’ ratings of the severity of multiple symptoms? Journal of Pain and Symptom Management, 40(2), 191–199.
28th Annual Conference of the International Society for Quality of Life Research. (2021). Quality of Life Research, 30(Suppl 1), 1–177.
El Miedany, Y., El Gaafary, M., Youssef, S., Ahmed, I., & Palmer, D. (2014). The arthritic patients’ perspective of measuring treatment efficacy: Patient Reported Experience Measures (PREMs) as a quality tool. Clinical and Experimental Rheumatology, 32(4), 547–552.
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., Filiberti, A., Flechtner, H., Fleishman, S. B., de Haes, J. C., et al. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85(5), 365–376.
Kaasa, S., Bjordal, K., Aaronson, N., Moum, T., Wist, E., Hagen, S., & Kvikstad, A. (1995). The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. European Journal of Cancer, 31(13–14), 2260–2263.
Bland, J. M., & Altman, D. G. (1997). Cronbach’s alpha. BMJ, 314(7080), 572.
Bartko, J. J. (1966). The intraclass correlation coefficient as a measure of reliability. Psychological Reports, 19(1), 3–11.
Fayers, P. M., & Machin, D. (2000). Quality of life: Assessment, analysis and interpretation. Sons Ltd.
Juniper, E. F., Guyatt, G. H., & Jaeschke, R. (1996). How to develop and validate a new health-related quality of life instrument. In B. Spilker (Ed.), Quality of Life and Pharmacoeconomics in Clinical Trials. Lippincott-Raven.
Selby, D., Cascella, A., Gardiner, K., Do, R., Moravan, V., Myers, J., & Chow, E. (2010). A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. Journal of Pain and Symptom Management, 39(2), 241–249.
Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174.
Xu, W., Dai, W., Gao, Z., Wang, X. S., Tang, L., Pu, Y., Yu, Q., Yu, H., Nie, Y., Zhuang, W., Qiao, G., Cleeland, C. S., & Shi, Q. (2022). Establishment of minimal clinically important improvement for patient-reported symptoms to define recovery after video-assisted thoracoscopic surgery. Annals of Surgical Oncology, 29(9), 5593–5604.
Minton, P. D., & Cohen, J. (1971). Statistical power analysis for the behavioral sciences. Journal of the American Statistical Association, 66, 334.
Boesen, V. B., Feldt-Rasmussen, U., Bjorner, J. B., Cramon, P. K., Grøenvold, M., Rasmussen, Å. K., & Watt, T. (2020). Shorter recall period for the thyroid-related patient-reported outcome measure ThyPRO did not change the accuracy as evaluated by repeated momentary measurements. Thyroid, 30(2), 185–191.
Qin, S., Nelson, L., McLeod, L., Eremenco, S., & Coons, S. J. (2019). Assessing test-retest reliability of patient-reported outcome measures using intraclass correlation coefficients: Recommendations for selecting and documenting the analytical formula. Quality of Life Research, 28(4), 1029–1033.
Yu, H., Yu, Q., Nie, Y., Xu, W., Pu, Y., Dai, W., Wei, X., & Shi, Q. (2021). Data quality of longitudinally collected patient-reported outcomes after thoracic surgery: Comparison of paper- and web-based assessments. Journal of Medical Internet Research, 23(11), e28915.
Funding
This research was funded by the National Key R&D Plan for Intergovernmental Cooperation, the Ministry of Science and Technology of China (Grant No. 2022YFE0133100).
Author information
Authors and Affiliations
Contributions
Study concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the abstract: Xueyao Su, Wei Xu, and Qiuling Shi. Revising the article critically for important intellectual content: Qiuling Shi and Wei Dai. Final approval of the version to be published: All authors. Statistical analysis: Xueyao Su, Wei Xu, Yang Pu, and Ruoyan Gong. Obtained funding: Qiuling Shi and Wei Dai. Administrative, technical, or material support: Qiuling Shi and Wei Dai. Study supervision: Qiuling Shi. Xueyao Su, and Qiuling Shi had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article. Authors are responsible for correctness of the statements provided in the manuscript.
Ethical approval
This study was approved by the Ethics Committee of Sichuan Cancer Hospital (Approval number: SCCHEC-02-2018-043).
Consent to participate
Informed consent was obtained from all participants included in the study. Each participant was aware that their private personal information would not be disclosed and were educated about the content and purpose of the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, X., Huang, Y., Gong, R. et al. Undergoing Lung Surgery (PSA-Lung) was appropriate for symptom assessment after discharge. Qual Life Res (2024). https://doi.org/10.1007/s11136-024-03636-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11136-024-03636-w