Abstract
Purpose
The objective of this study was to quantitatively evaluate psychological and quality of life-related complications at three months following discharge in hospitalized coronavirus disease 2019 (COVID-19) patients during the pandemic in Iran.
Methods
In this time-point analysis of prospective cohort study data, adult patients hospitalized with symptoms suggestive of COVID-19 were enrolled. Patients were stratified in analyses based on severity. The primary outcomes consisted of psychological problems and pulmonary function tests (PFTs) in the three months following discharge, with Health-related quality of life (HRQoL) as the secondary outcome. Exploratory predictors were determined for both primary and secondary outcomes.
Results
283 out of 900 (30%) eligible patients were accessible for the follow-up assessment and included in the study. The mean age was 53.65 ± 13.43 years, with 68% experiencing a severe disease course. At the time of the final follow-up, participants still reported persistent symptoms, among which fatigue, shortness of breath, and cough were the most common. Based on the regression-adjusted analysis, lower levels of forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio was associated with higher levels of depression (standardized β = − 0.161 (SE = 0.042), P = 0.017) and stress levels (standardized β =− 0.110 (SE = 0.047), P = 0.015). Furthermore, higher levels of anti-SARS-CoV-2 immunoglobulin-M (IgM) were associated with significantly lower levels of depression (standardized β = − 0.139 (SE = 0.135), P = 0.031).
Conclusions
There is an association between lung damage during COVID-19 and the reduction of pulmonary function for up to three months from acute infection in hospitalized patients. Varying degrees of anxiety, depression, stress, and low HRQoL frequently occur in patients with COVID-19. More severe lung damage and lower COVID-19 antibodies were associated with lower levels of psychological health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19) patients can present with varying disease severity and symptoms, ranging from asymptomatic to severe and fatal, resulting in more than 15% of those infected needing hospital admission [1]. Many COVID-19 patients admitted to the hospital have respiratory failure, and some require intensive care. The analysis of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) data recommend that some infected patients experienced long-term respiratory problems. Guidelines suggested structured respiratory follow-up of patients with clinic-radiological confirmation of COVID-19 pneumonia.
In addition to the high mortality rate, the disease has also caused severe psychological problems among patients [2].MERS and SARS survivors have been reported to experience significant psychological impairments, namely post-traumatic stress disorder (PTSD), anxiety, depression, and decreased health-related quality of life (HRQoL) for more than six months [3]. Similar to previous coronavirus outbreaks, patients’ HRQoL could be affected during and after covid-19 infection. Some studies have demonstrated that the survivors of COVID-19 are at a higher risk for physical and mental performance decline and that their pulmonary function remains impaired for months [4]. Nevertheless, the investigations conducted on COVID-19 patients could have been more thorough, leading to a better comprehension of the disease. Such information can assist in guaranteeing timely and efficacious management of many health complications to restore premorbid HRQoL [5]. Iran is among the countries with a higher COVID-19 prevalence, and half of the Iranians have been revealed to have restricted health literacy, which is more common in exposed groups, including homemakers, the unemployed, and those of older age [6]. As the largest, most populated city in central Iran, Isfahan was among the three top Iranian cities with the highest number of COVID-19 cases. Despite the numerous COVID-19 patients being cured and discharged in Iran, the pulmonary and extra-pulmonary sequela of COVID-19 following recovery from the acute disease still affect the patients. Considering the magnitude of the problem and the ongoing adverse effects influencing the vast number of patients, evaluating such problems and their relationship with acute disease features in different regions is necessary. The current study aimed to track the course of clinical outcomes in COVID-19 patients after discharge and identify potential risk factors to improve post-discharge management. To achieve said goals, we investigated the participants’ post-discharge health from various aspects, including lasting symptoms, HRQoL (physical and mental health), physical functionality/limitations, presence of pain, general health, vitality, social performance, and psychological and mental disorders in addition to assessing pulmonary function impairment and performing laboratory testing.
Methodology
Study design and participants
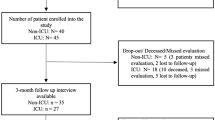
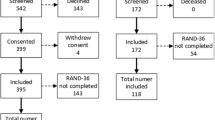
It is a time-point analysis using data from an open cohort study of COVID-19. Patients discharged beginning on March 1, 2020, were included and underwent a three months long follow-up. All participants were chosen from patients hospitalized in Khorshid hospital in Isfahan City, Isfahan Province, Iran. Patients were contacted and interviewed on the 1st and 4th weeks after discharge by phone and during the 12th weeks of follow-up after discharge [7] and attended an outpatient hospital clinic to undergo a clinical evaluation, laboratory testing, and complete questionnaires. We defined COVID-19 diagnosis based on the Chinese COVID-19 treatment guidelines, identification, and the WHO provisional advice [8]. The patients were included based on chest computed high-resolution CT (HRCT) scans compatible with COVID-19 or positive polymerase chain reaction (PCR). The final analysis included data on remaining symptoms and complications, as mentioned earlier, from 283 discharged patients (Fig. 1).
Collection of baseline information
Baseline characteristics included data concerning patients’ demographic features, medical history, educational status, chest computed tomographic (CT) scans, underlying chronic diseases, and clinical signs and symptoms extracted anonymously from electronic medical records.
Symptoms definitions
Imaging protocol
A 64-slice Philips scanner was used with a low dose protocol, without contrast, in participants with an oxygen saturation of > 60% and at full inspiration to obtain whole chest CT images. Two radiologists with significant experience in thorax imaging interpretation evaluated the images. Any disagreement was resolved by a senior researcher with more than a decade of experience in chest CT image interpretation. The features were documented for each CT scan, including the pattern, distribution (transverse and craniocaudal), and disease severity based on a semi-quantitative scoring system [9].
Severity definition
Eligible patients were divided into two groups concerning the severity of the disease. Based on clinical consensus and previous evidence [10], severe participants were defined as those meeting the following criteria: respiration rate (RR) ≥ 30 times/min, oxygen saturation level ≤ 88% in the resting position, and pulse rate ≥ 130/min.
Follow-up explanatory variables
Exclusion criteria
The exclusion criteria included: incomplete information on comorbidities and unwillingness to participate in the study. We also did not include patients with a history of significant co-morbid conditions such as pneumonia, interstitial lung disease, previous bronchial asthma, pulmonary neoplasm, sepsis, tuberculosis, and cystic fibrosis diagnosis.
Pulmonary function
Pulmonary function tests (PFTs) were performed 12 weeks after discharge to assess obstructive and restrictive pulmonary diseases and diffusion disorders. We measured forced expiratory volume (FEV1) and forced vital capacity (FVC) in one second. The Tiffeneau index (Ti) was calculated by dividing the FEV1 by the FVC to measure airway obstruction.
Serology test
Provided samples were also tested for anti-SARS-CoV-2 immunoglobulin-M (IgM) using the Pishtaz Teb ELISA kit. A sandwich ELISA kit detected the subjects’ serum SARS-CoV-2 antibodies (IgM and IgG). A 100 μL of diluted serum (1:100) was applied to a 96-well microplate (coated with N protein) and incubated at 37 °C for 1 h to detect IgM. After washing, the wells were filled with 100 μL of secondary antibodies (against human IgM) labeled with conjugate and incubated at 37 °C for 30 min. One hundred μL of the substrate was added to the wells and incubated at 37 °C for 15 min for the secondary wash cycle. Finally, the reaction was arrested by applying a stop solution to the wells. Within 30 min, each well’s optical density (OD) was measured using a microplate reader set to 450 nm. The antibody concentration was calculated as the ratio of OD to the cut-off value. The dilution factor was adjusted (1:20), and the cut-off value was changed (OD of the blank well + 0.15) to detect IgG. Figure 1 presents the study protocol in further detail.
Follow-up outcomes
HRQoL evaluation
We used SF-36 Standard Persian Version [11], which was translated from the International Quality of Life Assessment (IQOLA), comprising an individual item of health transition (HT) and 35 items. They were classified into eight subscales of physical function (PF), restrictions caused by physical health problems (RP, role physical), general health (GH), bodily pain (BP), vitality (VT), limitations caused by emotional health problems (RE, role-emotional), social functioning (SF), and mental health. Each domain was given a score of 0–100, with greater scores revealing a more satisfactory functional status [12]. A standard scoring algorithm was made for aggregating scores from the eight SF-36 subscales in two distinct, higher-order summary scores: Physical Component Summary (PCS) and Mental Component Summary (MCS) [13].
Psychiatric disorders
All patients were invited to fill out self-report screening questionnaires assessing psychological symptoms. Furthermore, patients were followed up by a clinical psychologist who evaluated psychological adjustment in a semi-structured clinical interview during which anxiety, depression, and post-traumatic stress symptoms were assessed.
In the 12th week after discharge, each patient attended the hospital to complete the Patient Health Questionnaire-9 (PHQ-9) and the Depression Anxiety Stress Scales (DASS-21) [14]. We used the self-report PHQ-9 questionnaire to measure the depression severity, with the total scores categorized as following: severe depression (15–21), moderate depression (10–14), mild depression (5–9), and no depression (0–4) [15]. The stress subscale was assessed using questions one, six, eight, eleven, twelve, fourteen, and eighteen. The total stress subscale scores were classified into five groups: extremely severe stress (35– 42), severe stress (27–34), moderate stress (19–26), mild stress (11–18), and normal (0–10). The anxiety subscale was estimated using question numbers two, four, seven, nine, fifteen, nineteen, and twenty. The anxiety subscale score was also divided into five groups: extremely severe anxiety (20–42), severe anxiety (15–19), moderate anxiety (10–14), mild anxiety (7–9), and normal (0–6).
Reporting and ethics
The hospital’s ethical review board approved the study protocol (Ethical Committee for COVID-19 related research at the Isfahan University of medical sciences; IR.MUI.RESEARCH.REC.1399.479); research data were securely stored, according to the General Data Protection Regulation. The experimental protocol was in concordance with the Declaration of Helsinki. Besides the oral explanation, all patients admitted to the hospital or a first-degree family member in those with severe circumstances were given an official letter asking permission for their data to be used for research purposes and informing them on opting out should they see fit. Informed consent with an opt-out option was obtained from all patients, and none of the admitted patients declined to participate. The study was approved (IR.MUI.MED.REC.1399.517) by the Isfahan University of Medical Sciences (IUMS) ethics committee.
Variables discrimination
A PHQ-9 total score of ≥ 5 was considered as “having depression,” while a total score of ≥ 10 and ≥ 15 were defined as “moderate depression” and “severe depression,” respectively [15]. The cut-offs for mental health consisted of ≤ 44, > 44, indicating weak and medium/good mental health, and for physical health score ≤ 43, > 43, indicating weak and medium/good physical health, respectively [16]. Based on clinical guidelines, the FEV1(%) and FVC (%) cut-offs were considered 80% to analyze the lung function parameters. However, in clinical practice, an FEV1 / FVC ratio of less than 0.7 has also been used [17].
Statistical analysis
The mean ± standard deviation was used for quantitative variables, and the frequency (percentage) was used for qualitative variables. Chi-square and independent t-tests were used for descriptive statistical analysis of categorical and quantitative data, respectively. The Mann–Whitney test was used for quantitative data that did not follow a normal distribution. Cochran’s Q-test was performed to compare the incidence of symptoms in patients in four follow-up stages. Crude and adjusted linear regression models reporting regression coefficients with a 95% confidence interval (adjusted for potential confounders including sex, age, smoking status, education, comorbidities, NIV and oxygen mask use, and length of stay) were used to determine the association between different patient’s characteristics and continuous outcomes (depression, anxiety, and post-traumatic stress symptoms). Univariates and multivariate binary logistic regression reporting odds ratio (%95 CI) were used to determine the factors associated with quality of life components (PCS and MCS). The same strategy was used with confounders in the linear regression models. Cramer’s V and Hedges’ g were used to estimate the effect size for the independent-sample t-test and Chi-square tests for comparison between patients included in the analysis and drop-out patients. P values < 0.05 were considered significant. All analyses were done with STATA software version 14.2 (Stata Corp, College Station, Tex).
Results
Of the 900 patients admitted to the hospital from January 1 to April 30, 2020, 350 who were discharged and accepted to participate in the follow-up step were recruited in this study. Of those, 27 were excluded because of chronic respiratory disease, and 40 patients (11% of eligible patients) did not participate in 12th-week follow-up for pulmonary function tests, quality of life assessments, and measuring psychiatric outcomes. Data from 283 hospitalized patients accessible for the 12th follow-up assessment were analyzed (Fig. 1). No significant difference existed between participants who remained in the study and those who dropped out (Online Resource (1). Of the 283 patients, 33 (11%) were admitted to ICU. Table 1 shows a descriptive analysis of discharged patients, while Tables 2 and 3 provide the descriptive analysis at the 12th week of follow-up in the severe and non-severe groups. The majority were male (62.19%), nonsmokers (88.69%), had up to high-school education (36.04%), and the mean BMI was 28.33 ± 4.80. The most common symptoms among patients at the time of hospitalization were fever (75.97%), cough (73.5%), and shortness of breath (64.66%). Comparing the symptom distribution between the two groups of severity showed significant differences in the case of fever, shortness of breath, and sneezing (P < 0.05) (Table 1). Figure 2 shows the distribution of 8 common symptoms at different follow-up points between patients with severe and non-severe conditions. The results of Cochran’s Q test show the decreasing trend of seven symptoms from hospitalization to the 12th week, except for the chest pain (P = 0.250), which does not show a significant difference in the four follow-up points.
Lung function
Mild pulmonary function abnormalities were present in less than 10% of patients during follow-up, which might be explained by the persistent cough and shortness of breath symptoms observed in some patients after discharge (Fig. 2).
The mean age was (53.65 ± 13.43) years old, and 195 participants had a severe disease course. Pulmonary function impairment was seen at rates of 4.24% and 18.73% as measured by low FEV1/FVC and low FVC, respectively. There was no significant difference based on the FEV1/FVC criteria between the discharged survivors with different disease severity (P > 0.05), but a significant difference (P < 0.05) concerning other pulmonary function measures (e.g., FEV1, FVC) was observed.
Furthermore, the results showed a significant difference in diffusing-capacity measurements between the severe and non-severe groups. The two severity groups differed in length of hospitalization (9.07 ± 6.24 for the severe patients, vs. 5.24 ± 3.47 for the patients with the non-severe condition, P < 0.001), and use of oxygen masks (18.46% in severe patients vs. 3.41% in non-severe condition) (Table 2).
Mental health
Table 3 presents depressive, anxiety, and post-traumatic stress symptoms in the sample. Clinically significant levels of depressive, anxiety, and post-traumatic stress symptoms were reported by 5.16%, 4.95%, and 7.62% of the sample.
Table 4 presents the results of the linear regression models of the respiratory functions, serology results, and outcomes (depression, anxiety, stress, and HRQoL components) adjusted by probable confounders, including demographic variables, comorbidities, severity, using NIV or oxygen mask, and length of hospital stay.
On a linear, fully-adjusted regression model, lower levels of FEV1/FVC were associated with higher levels of depression (standardized β = − 0.161 (SE = 0.042), P = 0.017) and stress level (standardized β = − 0.110 (SE = 0.047), P = 0.015), respectively. Furthermore, a higher level of IgM was associated with significantly lower levels of depression (standardized β = − 0.139 (SE = 0.135), P = 0.031) similar to the level of depression and IgM in the crude model (standardized β = − 0.153 (SE = 0.141), P = 0.021). The crude regression model (Table 4) also shows that it significantly reduces the amount of FEV1 and leads to higher levels of depression, anxiety, and stress at the level of 0.01 in patients, but in the adjusted model, there is no significant difference (P > 0.05). However, oxygen consumption at home had not affected the patient’s level of anxiety, depression, and stress. The presence of comorbidity was strongly associated with the incidence of depression (P < 0.05).
Health-related quality of life (HRQoL)
The mean scores of the PCS and MCS domains were 35.70 ± 8.29 and 46.23 ± 16.64 for severe, respectively, and 39.64 ± 17.42 and 48.83 ± 22.71 for non-sever conditions, respectively.
The results for two components of HRQoL showed that the mental component summary was significantly negatively associated with a higher level of IGG (OR = 0.27 P = 0.001) and FEV (%) (OR = 0.40 P < 0.001) according to the fully-adjusted model. Moreover, the normal FEV1/FVC ratio was associated with a good physical component level (OR = 2.42 P < 0.001). Moreover, the PCS score after controlling the covariates was positively associated with higher levels of both FEV (%) (OR = 2.95 P = 0.003) and FVC (%) (OR = 3.50 P < 0.01), respectively (Table 5).
Discussion
The COVID-19 pandemic has led to a growing population recovering from severe acute respiratory syndrome infection. The sequela of COVID-19 in those recovering from acute infection is uncertain. However, limited observational studies suggest that these patients may experience a broad range of symptoms after recovery from the acute phase of the novel coronavirus [18,19,20,21,22]. The current study describes the prevalence of symptoms, pulmonary function, quality of life, and psychological disorders in severe and non-severe patients with COVID-19 three months following hospital discharge.
Persistent symptoms
We found that at 12 weeks after hospital discharge, fatigue, cough, shortness of breath, and chest pain were the main symptoms of patients who had recovered from COVID-19. In addition, our study showed that most patients with severe COVID-19 had more residual symptoms than non-severe individuals. Our results are comparable to recent studies: a study by Arnold et al., which evaluated 110 patients with COVID-19, found that the most common symptoms remaining in patients at 12 weeks were fatigue, shortness of breath, and cough [23]. Nevertheless, the causality and pathogenesis of fatigue after COVID-19 are unclear [24]. According to previous evaluations of SARS, pulmonary dysfunction, myositis, immune-mediated inflammation, and treatment complications could impact the condition’s progress [25,26,27,28,29].
Furthermore, the remaining symptoms appear to last longer. Huang et al. showed that in approximately 50% of individuals, at least one of the symptoms lasts up to 12 months in the long-term follow-up of COVID-19 patients [24]. These results provide practical information for physicians caring for those recovering from COVID-19 disease. Moreover, rehabilitation and proper recommendations for managing such patients should be considered.
Pulmonary function
The optimal timing to obtain PFTs in patients recovering from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is uncertain. Data on pulmonary function following the COVID-19 pandemic are expanding, and the studies suggest that pulmonary function abnormalities may persist, particularly among those with severe illness [24, 30]. In a prospective cohort study including 103 patients, approximately half of all participants had persistent dyspnea, and one in four had reduced DLCO detected three months after hospital admission due to COVID-19. In addition, they showed a significant reduction in the FEV1 / FVC ratio in patients admitted to the ICU [31]. In this 3-month follow-up, we recorded lower lung volumes in patients recovering from COVID-19. At the follow-up, a restrictive pattern (FVC < 80%) was observed in 22% and 11% of patients with severe and non-severe conditions, respectively, which is comparable to the results of previous studies [31, 32].
Furthermore, long-term follow-up has shown improvement in PFTs and pulmonary volumes at 6 and 12 months [24, 30]. In addition, we observed a significant decrease in lung volumes (FEV1 and FVC) in patients with severe conditions compared to non-severe individuals, as shown in previous studies [33, 34]. The similar FEV1/FVC ratio in severe and non-severe patients suggests a tendency for a restrictive pattern. It is of note that underlying conditions can be risk factors in lung function reduction. In a study that included 219 patients who survived COVID-19, A history of underlying diseases such as CKD and diabetes was associated with pulmonary function impairment [35]. We also observed that the prevalence of hypertension and diabetes is significantly higher in patients with severe COVID-19. Therefore, we recommend that post-COVID-19 patients with underlying diseases be followed up more carefully.
Health-related quality of life (HRQoL)
Like many other aspects, it seems that the patients’ HRQoL is affected during and post-COVID-19 infection. Assessing the quality of life of those affected by COVID-19 and examining the factors that cause it are crucial in determining the disease burden in each region. Whereas the long-term impacts of COVID-19 are not well understood, the substantial impact of COVID-19 on the physical and mental dimensions of HRQoL has been shown [4, 36]. A limited number of studies have demonstrated that patients recovering from acute COVID-19 are at increased risk for psychiatric disorders [22]. For example, in the study by Xie et al., 153 848 patients who recovered from acute COVID-19 showed an increased risk of incident anxiety disorders, depressive disorders, and stress [37].
Furthermore, based on previous evidence in SARS and MERS, it has been demonstrated that the effect of COVID-19 on depression can be similar [38]. Overall, we know COVID-19 is associated with an increased risk of neurological and psychiatric outcomes. However, the mechanisms of the increased risks are not entirely clear [39]. It is of note that suggested mechanisms for this association include viral invasion of CNS, dysregulated immune response on CNS, and hypercoagulable states [40,41,42]. In addition, indirect mechanisms, including loss of employment, financial problems, and loneliness, could also affect mental health [43]. Studies conducted before the COVID-19 pandemic showed that obstructive and restrictive lung function is associated with significantly increased mental health problems [44] and that patients with bronchiectasis may experience elevated levels of anxiety and depression [45]. Therefore, in this study, we investigated the effect of PFTs on psychological disorders and found lower levels of FEV1/FVC ratio to be associated with higher levels of depression and stress in COVID-19 patients. These results are based on linear fully-adjusted regression analysis. However, the mechanism of the association between PFTs and psychological problems is not known.
Additionally, it was found that pulmonary function can independently affect physical and mental aspects of quality of life (PCS and MCS) and that patients with better pulmonary function (based on FEV1%, FVC%, and FEV1/FVC ratio) had a higher HRQoL score.
Furthermore, it has been demonstrated that antibody response is much greater in severe COVID-19 patients than in mild patients [46]. We also showed that the serum SARS-CoV-2 antibodies (IgG) level in patients with severe conditions is significantly higher at 3-month follow-up. It is worth mentioning that antibodies may also be as dangerous as they are helpful, as, despite the importance of antibody response, they can also cause tissue damage in patients [47]. However, whether this tissue damage can cause pulmonary dysfunction has yet to be determined. Nevertheless, we have found higher levels of IgM to be associated with significantly lower levels of depression. However, the mechanism of the association between SARS-CoV-2 IgM and psychological problems is still not known. In addition, it was found that immune response and higher IgG serum levels can be associated with higher HRQoL scores (both PCS and MCS). In COVID-19, several connections between physical and mental conditions could affect the quality of life and should be considered when dealing with patients.
While we provide evidence of the significant impact of COVID-19 on pulmonary function, quality of life, and mental health, this study has limitations. Firstly, our study is a single institution study. Furthermore, only critically ill patients admitted to hospitals were included. Thus, the results may not reflect the experience and results of critically ill COVID-19 survivors of other hospitals in other regions. Additionally, at the time of the study, the COVID-19 alpha variant was dominant among patients, and the impact of other variants of COVID-19, such as delta and omicron, could be different. Another limitation is the lack of a pre-COVID-19 assessment before hospitalization.
Conclusions
The severity of COVID-19 has a long-term effect on the reduction of pulmonary function, and the severity of the initial disease has many effects on the quality of life and mental health, directly or indirectly. Therefore, it is recommended that in the follow-up of the patients hospitalized due to COVID-19, people with the more severe disease be evaluated in terms of pulmonary function and that all patients be assessed in terms of quality of life and mental health.
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Lee, A. (2020). Wuhan novel coronavirus (COVID-19): Why global control is challenging? Public Health, 179, A1-2.
Xiang, Y. T., Yang, Y., Li, W., et al. (2020). Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry, 7(3), 228–229.
Lai, C. C., Shih, T. P., Ko, W. C., Tang, H. J., & Hsueh, P. R. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents, 55(3), 105924.
Gamberini, L., Mazzoli, C. A., Sintonen, H., Colombo, D., Scaramuzzo, G., Allegri, D., Tonetti, T., Zani, G., Capozzi, C., Giampalma, E., Agnoletti, V., Becherucci, F., Bertellini, E., Castelli, A., Cappellini, I., Cavalli, I., Crimaldi, F., Damiani, F., Fusari, M., … Spadaro, S. (2021). ICU-RER COVID-19 Collaboration. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Quality of Life Research, 30(10), 2805–2817. https://doi.org/10.1007/s11136-021-02865-7
Mirzaei, N., Jahanian Sadatmahalleh, S., Bahri Khomami, M., Moini, A., & Kazemnejad, A. (2021). Sexual function, mental health, and quality of life under strain of COVID-19 pandemic in Iranian pregnant and lactating women: A comparative cross-sectional study. Health and Quality of Life Outcomes, 19, 66.
Montazeri, A.L.I., Tavousi, M., Rakhshani, F., Azin, S.A., Jahangiri, K., Ebadi, M., Naderimagham, S., Solimanian, A., Sarbandi, F., Motamedi, A., & Sistani, M.M.N. (2014). Health Literacy for Iranian Adults (HELIA): Development and psychometric properties. Payesh (Health Monitor), 13(5), 589–599. http://payeshjournal.ir/browse.php?a_id=279&sid=1&slc_lang=en
Sami, R., Soltaninejad, F., Amra, B., Naderi, Z., Haghjooy Javanmard, S., Iraj, B., Haji Ahmadi, S., Shayganfar, A., Dehghan, M., Khademi, N., Sadat Hosseini, N., Mortazavi, M., Mansourian, M., Mañanas, M. A., Marateb, H. R., & Adibi, P. (2020). A one-year hospital-based prospective COVID-19 open-cohort in the Eastern Mediterranean region: The Khorshid COVID Cohort (KCC) study. PLoS ONE, 15(11), e0241537.
Li, J., Zhang, K., Bao, J., Yang, J., & Wu, C. (2022). Potential mechanism of action of Jing Fang Bai Du San in the treatment of COVID-19 using docking and network pharmacology. International Journal of Medical Sciences, 19(2), 213–224.
Ai, T., Yang, Z., Hou, H., Zhan, C., Chen, C., Lv, W., et al. (2020). Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology, 296, 32–40.
Sami, R., Soltaninejad, F., Shayganfar, A., Mirfendereski, S., Mansourian, M., Khademi, N., Dehghan, M., Khorrami, Z., Jalali, S., & Mokhtari, Z. (2022). Severity of disease and COVID-19 complications during hospital stay: A prospective cohort study. Archives of Iranian Medicine, 25(6), 383–393.
Montazeri, A., Goshtasebi, A., Vahdaninia, M., & Gandek, B. (2005). The Short Form Health Survey (SF-36): Translation and validation study of the Iranian version. Quality of Life Research, 14(3), 875–882. https://doi.org/10.1007/s11136-004-1014-5
Ell, K., Unützer, J., Aranda, M., Sanchez, K., & Lee, P.-J. (2005). Routine PHQ-9 depression screening in home health care: Depression, prevalence, clinical and treatment characteristics and screening implementation. Home Health Care Services Quarterly, 24, 1–19.
Taft, C., Karlsson, J., & Sullivan, M. (2001). Do SF-36 summary component scores accurately summarize subscale scores? Quality of Life Research, 10(5), 395–404. https://doi.org/10.1023/a:1012552211996
Nguyen, H. C., Nguyen, M. H., Do, B. N., et al. (2020). People with suspected covid-19 symptoms were more likely depressed and had lower health-related quality of life: the potential benefit of health literacy. Journal of Clinical Medicine, 9(4), 965. https://doi.org/10.3390/jcm9040965
Kroenke, K., Spitzer, R. L., Williams, J. B., & Löwe, B. (2010). The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. General Hospital Psychiatry, 32(4), 345–359. https://doi.org/10.1016/j.genhosppsych.2010.03.006
Tapak, L., Cheraghi, F., Sadeghi, A., Shirmohammadi, N., & Feizybarnaji, A. (2022). Usefulness of the SF-36 Health Survey questionnaire in screening for health-related quality of life among parents of children with cancer: Latent profile analysis. Journal of Preventive Medicine and Hygiene, 63(1), 142–151. https://doi.org/10.15167/2421-4248/jpmh2022.63.1.2279
Singh, D., Agusti, A., Anzueto, A., Barnes, P. J., Bourbeau, J., Celli, B. R., Criner, G. J., Frith, P., Halpin, D. M. G., Han, M., López Varela, M. V., Martinez, F., Montes de Oca, M., Papi, A., Pavord, I. D., Roche, N., Sin, D. D., Stockley, R., Vestbo, J., … Vogelmeier, C. (2019). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. European Respiratory Journal, 53(5), 1900164. https://doi.org/10.1183/13993003.00164-2019
Zhao, Y. M., Shang, Y. M., Song, W. B., et al. (2020). Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine, 25, 100463.
Shah, A. S., Wong, A. W., Hague, C. J., et al. (2020). A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. https://doi.org/10.1136/thoraxjnl-2020-216308
Carfì, A., Bernabei, R., & Landi, F. (2020). Gemelli against COVID-19 post-acute care study group persistent symptoms in patients after acute COVID-19. JAMA, 324(6), 603–605. https://doi.org/10.1001/jama.2020.12603
Barman, M. P., Rahman, T., Bora, K., & Borgohain, C. (2020). COVID-19 pandemic and its recovery time of patients in India: A pilot study. Diabetes & Metabolic Syndrome, 14(5), 1205–1211. https://doi.org/10.1016/j.dsx.2020.07.004
Xiong, Q., Xu, M., Li, J., Liu, Y., Zhang, J., Xu, Y., & Dong, W. (2021). Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clinical Microbiology and Infection, 27(1), 89–95. https://doi.org/10.1016/j.cmi.2020.09.023
Arnold, D. T., Hamilton, F. W., Milne, A., Morley, A. J., Viner, J., Attwood, M., Noel, A., Gunning, S., Hatrick, J., Hamilton, S., Elvers, K. T., Hyams, C., Bibby, A., Moran, E., Adamali, H. I., Dodd, J. W., Maskell, N. A., & Barratt, S. L. (2021). Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax, 76(4), 399–401. https://doi.org/10.1136/thoraxjnl-2020-216086
Huang, L., Yao, Q., Gu, X., Wang, Q., Ren, L., Wang, Y., Hu, P., Guo, L., Liu, M., Xu, J., Zhang, X., Qu, Y., Fan, Y., Li, X., Li, C., Yu, T., Xia, J., Wei, M., Chen, L., … Cao, B. (2021). 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet, 398(10302), 747–758. https://doi.org/10.1016/S0140-6736(21)01755-4
Hui, D. S., Wong, K. T., Ko, F. W., Tam, L. S., Chan, D. P., Woo, J., & Sung, J. J. (2005). The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest, 128(4), 2247–2261. https://doi.org/10.1378/chest.128.4.2247
Ngai, J. C., Ko, F. W., Ng, S. S., To, K. W., Tong, M., & Hui, D. S. (2010). The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology, 15(3), 543–550. https://doi.org/10.1111/j.1440-1843.2010.01720.x
Hui, D. S., Joynt, G. M., Wong, K. T., Gomersall, C. D., Li, T. S., Antonio, G., Ko, F. W., Chan, M. C., Chan, D. P., Tong, M. W., Rainer, T. H., Ahuja, A. T., Cockram, C. S., & Sung, J. J. (2005). Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax, 60(5), 401–409. https://doi.org/10.1136/thx.2004.030205
Su, M. C., Hsieh, Y. T., Wang, Y. H., Lin, A. S., Chung, Y. H., & Lin, M. C. (2007). Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome. Respiration, 74, 511–516.
Tsai, L. K., Hsieh, S. T., Chao, C. C., et al. (2004). Neuromuscular disorders in severe acute respiratory syndrome. Archives of Neurology, 61, 1669–1673.
Huang, C., Huang, L., Wang, Y., Li, X., Ren, L., Gu, X., Kang, L., Guo, L., Liu, M., Zhou, X., Luo, J., Huang, Z., Tu, S., Zhao, Y., Chen, L., Xu, D., Li, Y., Li, C., Peng, L., … Cao, B. (2021). 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet, 397(10270), 220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Lerum, T. V., Aaløkken, T. M., Brønstad, E., Aarli, B., Ikdahl, E., Lund, K. M. A., Durheim, M. T., Rodriguez, J. R., Meltzer, C., Tonby, K., Stavem, K., Skjønsberg, O. H., Ashraf, H., & Einvik, G. (2021). Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. European Respiratory Journal, 57(4), 2003448. https://doi.org/10.1183/13993003.03448-2020
Ramani, C., Davis, E. M., Kim, J. S., Provencio, J. J., Enfield, K. B., & Kadl, A. (2021). Post-ICU COVID-19 Outcomes: A case series. Chest, 159(1), 215–218. https://doi.org/10.1016/j.chest.2020.08.2056
Guler, S. A., Ebner, L., Aubry-Beigelman, C., Bridevaux, P. O., Brutsche, M., Clarenbach, C., Garzoni, C., Geiser, T. K., Lenoir, A., Mancinetti, M., Naccini, B., Ott, S. R., Piquilloud, L., Prella, M., Que, Y. A., Soccal, P. M., von Garnier, C., & Funke-Chambour, M. (2021). Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. European Respiratory Journal, 57(4), 2003690. https://doi.org/10.1183/13993003.03690-2020
de Graaf, M. A., Antoni, M. L., Ter Kuile, M. M., Arbous, M. S., Duinisveld, A. J. F., Feltkamp, M. C. W., Groeneveld, G. H., Hinnen, S. C. H., Janssen, V. R., Lijfering, W. M., Omara, S., Postmus, P. E., Ramai, S. R. S., Rius-Ottenheim, N., Schalij, M. J., Schiemanck, S. K., Smid, L., Stöger, J. L., Visser, L. G., … Roukens, A. H. E. (2021). Short-term outpatient follow-up of COVID-19 patients: A multidisciplinary approach. EClinicalMedicine, 32, 100731. https://doi.org/10.1016/j.eclinm.2021.100731
Bellan, M., Soddu, D., Balbo, P. E., Baricich, A., Zeppegno, P., Avanzi, G. C., Baldon, G., Bartolomei, G., Battaglia, M., Battistini, S., Binda, V., Borg, M., Cantaluppi, V., Castello, L. M., Clivati, E., Cisari, C., Costanzo, M., Croce, A., Cuneo, D., … Pirisi, M. (2021). Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Network Open, 4(1), e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
Gamberini, L., Mazzoli, C. A., Prediletto, I., Sintonen, H., Scaramuzzo, G., Allegri, D., Colombo, D., Tonetti, T., Zani, G., Capozzi, C., Dalpiaz, G., Agnoletti, V., Cappellini, I., Melegari, G., Damiani, F., Fusari, M., Gordini, G., Laici, C., Lanza, M. C., … Spadaro, S. (2021). Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respiratory Medicine, 189, 106665. https://doi.org/10.1016/j.rmed.2021.106665
Xie, Y., Xu, E., & Al-Aly, Z. (2022). Risks of mental health outcomes in people with covid-19: Cohort study. BMJ, 16(376), e068993. https://doi.org/10.1136/bmj-2021-068993
Rogers, J. P., Chesney, E., Oliver, D., Pollak, T. A., McGuire, P., Fusar-Poli, P., Zandi, M. S., Lewis, G., & David, A. S. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry, 7(7), 611–627. https://doi.org/10.1016/S2215-0366(20)30203-0
Taquet, M., Geddes, J. R., Husain, M., Luciano, S., & Harrison, P. J. (2021). 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry, 8(5), 416–427. https://doi.org/10.1016/S2215-0366(21)00084-5
Yang, A. C., Kern, F., Losada, P. M., Agam, M. R., Maat, C. A., Schmartz, G. P., Fehlmann, T., Stein, J. A., Schaum, N., Lee, D. P., Calcuttawala, K., Vest, R. T., Berdnik, D., Lu, N., Hahn, O., Gate, D., McNerney, M. W., Channappa, D., Cobos, I., … Wyss-Coray, T. (2021). Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature, 595(7868), 565–571. https://doi.org/10.1038/s41586-021-03710-0
Panigada, M., Bottino, N., Tagliabue, P., Grasselli, G., Novembrino, C., Chantarangkul, V., Pesenti, A., Peyvandi, F., & Tripodi, A. (2020). Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. Journal of Thrombosis and Haemostasis, 18(7), 1738–1742. https://doi.org/10.1111/jth.14850
Meinhardt, J., Radke, J., Dittmayer, C., Franz, J., Thomas, C., Mothes, R., Laue, M., Schneider, J., Brünink, S., Greuel, S., Lehmann, M., Hassan, O., Aschman, T., Schumann, E., Chua, R. L., Conrad, C., Eils, R., Stenzel, W., Windgassen, M., … Heppner, F. L. (2021). Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience, 24(2), 168–175. https://doi.org/10.1038/s41593-020-00758-5
Townsend, E. (2020). COVID-19 policies in the UK and consequences for mental health. Lancet Psychiatry, 7(12), 1014–1015. https://doi.org/10.1016/S2215-0366(20)30457-0
Goodwin, R. D., Chuang, S., Simuro, N., Davies, M., & Pine, D. S. (2007). Association between lung function and mental health problems among adults in the United States: Findings from the first national health and nutrition examination survey. American Journal of Epidemiology, 165(4), 383–388. https://doi.org/10.1093/aje/kwk026
O’Leary, C. J., Wilson, C. B., Hansell, D. M., Cole, P. J., Wilson, R., & Jones, P. W. (2002). Relationship between psychological well-being and lung health status in patients with bronchiectasis. Respiratory Medicine, 96(9), 686–692. https://doi.org/10.1053/rmed.2002.1330
Liu, L., To, K. K., Chan, K. H., Wong, Y. C., Zhou, R., Kwan, K. Y., Fong, C. H., Chen, L. L., Choi, C. Y., Lu, L., Tsang, O. T., Leung, W. S., To, W. K., Hung, I. F., Yuen, K. Y., & Chen, Z. (2020). High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerging Microbes & Infections, 9(1), 1664–1670. https://doi.org/10.1080/22221751.2020.1791738
Wang, E. Y., Mao, T., Klein, J., Dai, Y., Huck, J. D., Jaycox, J. R., Liu, F., Zhou, T., Israelow, B., Wong, P., Coppi, A., Lucas, C., Silva, J., Oh, J. E., Song, E., Perotti, E. S., Zheng, N. S., Fischer, S., Campbell, M., & Ring, A. M. (2021). Diverse functional autoantibodies in patients with COVID-19. Nature, 595(7866), 283–288. https://doi.org/10.1038/s41586-021-03631-y
Acknowledgements
The authors are grateful to the physicians and nurses of the participating hospitals.
Funding
Isfahan University of Medical Sciences, IR.MUI.MED.REC.1399.517, Ramin Sami
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sami, R., Arabi, S., Ghasemi, K. et al. Post-discharge health assessment in survivors of coronavirus disease: a time-point analysis of a prospective cohort study. Qual Life Res 32, 2681–2693 (2023). https://doi.org/10.1007/s11136-023-03415-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03415-z