Abstract
Grapefruit peel contains a high concentration of naringin- a potent antioxidant with strong bioactive properties. In this study, a new type of functional chocolate fortified with grapefruit peel extract and different concentrations of aqueous methanol and ethanol were evaluated as extraction solvents. A new high-performance liquid chromatography (HPLC) method to analyze the naringin content of the fortified chocolates was developed with a recovery of 107% ± 3.1% and repeatability below 3.5%. A sensory evaluation was conducted to assess the preference for the chocolates among individuals who self-described a preference for bitter flavors. No significant preference was observed in the cases of astringency and aftertaste while the increased bitterness proved to be favorable. However, taste, flavor and overall acceptability were regarded somewhat less favorably. While chocolate proved to be a satisfactory carrier for naringin and had several enjoyable characteristics, further research may focus on improving the organoleptic properties of chocolates fortified by naringin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naringin is a polyphenol and potent antioxidant abundant within the peels of grapefruit (Citrus paradisi), pomelo (Citrus grandis), and sour orange (Citrus aurantium). The molecule is a flavanone-7-O-glycoside between a naringenin aglycone and a neohesperidose moiety [1]. This compound has been shown to protect against neurodegenerative conditions such as Alzheimer’s disease [2] cardiovascular diseases, and diabetes [3] while also exhibiting hepatoprotective, anti-obesity [4] nephroprotective [5, 6] anti-inflammatory [7]and anti-carcinogenic characteristics [8, 9]. Naringin is generally recognized as safe [1] and no significant toxic effects have been found [9]. Additionally, the NOAEL of naringin was found to be > 500 mg/kg of body weight when tested on Beagle dogs [10]. However, it is important to note that ingesting naringin- after being hydrolyzed into naringenin- can cause adverse drug interactions. Thus, the FDA has begun to put warning labels on certain medications calling their users to avoid grapefruit products [11].
Research into foods fortified with polyphenols is rapidly increasing due to their availability and prophylactic activity. Given the aforementioned bioactive properties, grapefruit peel and naringin have been used as an ingredient in a several types of foodstuff such as cakes [12, 13], cookies [14, 15] mustard oil [16], margarine beverages [17]. Additionally, several studies have evaluated the potential of citrus peel as a functional food ingredient [18, 19]. However, to the best of our knowledge, grapefruit peel has not been utilized as a functional ingredient in chocolate. Dark chocolate may well serve as an excellent carrier for plant extracts because it can easily mask its taste and color while allowing for even distribution within the food structure. Additionally dark chocolate has a rich phenolic profile by itself, containing large quantities of flavan-3-ols such as catechin and epicatechin. As with naringin, these compounds also have anti-inflammatory properties and attenuate risk factors for cardiovascular disease [20, 21]. For this reason, the goal of this study was to produce chocolate fortified with grapefruit peel extract and to develop a novel HPLC method for analyzing the naringin content of the product.
Materials and Methods
The materials and methods used in this work are reported in detail in the Supplemental Material section.
Results and Discussion
HPLC-Method Validation
To validate our method, we evaluated the resolution, number of theoretical plates, retention time, and peak asymmetry of the method by injecting three replicate standards and chocolate extracts. The determination coefficient (R2) of the calibration curve of naringin was 0.9999, which indicates the linearity of the HPLC method. The LOQ was found to be 4 µg/mL, while the LOD was estimated to be 1.2 µg/mL. Recovery was determined to be 107% ± 3.1% while the repeatability and reproducibility of our method was evaluated by examining the relative standard deviation (RSD%) of naringin within the chocolate. The repeatabilities (intra-day precisions) determined by triplicate measurements on four various days were 3.7, 0.4, 3.5, and 0.7%. The reproducibility (inter-day precision) calculated from 4 triplicate measurements (twelve replicates) was found to be 3.5%. Sensitivity was recorded as 6.586 mL/µg. Therefore, these results indicate that the used method was adequate for the quantitative and qualitative determination of naringin in chocolate.
Effect of the Solvent Composition on the Extraction Yield
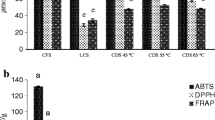
To determine the most effective solvent for the extraction of naringin from grapefruit peel, we made triplicate measurements of wet grapefruit peel extracts prepared immediately before injection. While doing so, the solvent composition was varied. As seen in Fig. 1, solvent compositions of 25, 50, 75, and 100% aqueous ethanol and methanol were tested. A visualization of our results can be seen in Fig. 1.
Confidence intervals refer to standard deviation. Lettering shows which solvents produced statistically identical outcomes.
As shown in Fig. 1, pure methanol was proven to be the best extraction solvent while pure ethanol and deionized water yielded the lowest concentration of naringin. However, methanol is well-known to be highly toxic and the aqueous solvents were difficult to remove from the extract. Therefore, ethanol was chosen as an extraction solvent for the production of chocolates used for sensory evaluation. The observed superior solvency of methanol was in line with the findings of Feng et al. [22] where the extraction efficiency of methanol and ethanol for naringin from oranges were compared. However, ethanol is still commonly used due to its nature as a food-grade solvent and overall effectiveness [23].
Characterization of Grapefruit peel Extract
The grapefruit peel had a dry matter content of 22.3% which was determined gravimetrically. The grapefruit peel extract was a dull, red-orange powder comprising 61.9%, 57.9%, and 42.95% of dry peel mass when extracted with methanol, ethanol, and 75% ethanol respectively. The measured naringin content of the dry extract was 130 ± 2.3 mg/g when using ethanol, 111.9 ± 1.6 mg/g when using methanol, and 96.2 ± 1.4 mg/g when using 75% ethanol. The extraction yield from the dry peel was 54.5 mg/g with methanol, 45.6 mg/g with ethanol, and 53.6 mg/g with 75% ethanol. This was in line with the findings of Li et al. [24] (52.03 mg/g when using methanol) showing that our extraction method provided sufficient yield. As highlighted by Sharma et al. [9], and Li et al. [25], naringin poses no health risks below 2 g per person or in a dose of less than 200 mg/kg in body weight.
HPLC Analysis of Fortified Chocolate
The protocol for chocolate production and analysis was based on the method used by Sik et al. [26]. Naringin and naringenin were both detected at 280 nm during the procedure. Our experiments showed that pure methanolic extraction resulted in the highest naringin yield (54.5 mg/g) from grapefruit peel so methanol was chosen for the extraction of naringin from the fortified chocolate. Methanolic solvents are commonly used to extract polyphenols from chocolate matrices as seen in studies by Belščak-Cvitanović et al. [27], Poliński et al. [28], and Carvalho et al. [29]. Fig. 2 shows the chromatogram measured for chocolates and the standard solution.
As seen from the chromatogram, naringin was successfully separated from the other minor components of the fortified chocolates. In addition, the measured concentration was 1.04 mg/g with 101 ± 3.4% recovery. This data highlights that dark chocolate provides a suitable matrix for fortification with naringin. Other authors have also examined the potential for chocolates to be fortified with fruit extracts offering proof of the suitability of chocolate to serve as a medium for functional ingredients. For example, Kaur et al. [30] produced a meat chocolate fortified with blueberry and raspberry extract and observed improved microbiological quality and boosted antioxidant activity. Polinski et al. [31] also measured an increase in phenolic content and antioxidant capacities when making dark chocolates fortified with chokeberry, elder flower, and elderberry extracts.
Effect of Extraction time on Naringin Yield
The amount of naringin that could be extracted from the chocolates as a function of time was also investigated. We also investigated the factor of extraction time during the extraction of naringin from chocolate. As seen in Fig. 3, extractions of 15, 30, 45, and 60 min provided a statistically identical result.
Results of Organoleptic Evaluation
Due to the strong change in the organoleptic properties caused by the fortification with grapefruit peel extract, the sensory evaluation was focused towards individuals who enjoy bitter flavors. However, the evaluators showed no significant preference for either chocolate in the categories of aftertaste, or astringency. In fact, a clear preference was shown for the enhanced bitterness of the chocolate. However, taste, flavor and overall acceptability was greater in the case of the control chocolates. A summary of the organoleptic trials can be seen in Fig. 4.
There was no significant difference found within the evaluation of the visible traits of the chocolate. Even though the increase in bitterness may be welcome for some, further research may focus on improving the sensory characteristics of fortified chocolates. A possible solution to this issue may be using encapsulation technologies, which may decrease the changes in flavor while maintaining or increasing the antioxidant activity and bioavailability of naringin [32, 33]. Alternatively, naringin may be hydrolyzed to its less-bitter aglycone moiety naringenin through the use of naringinase, or a combination of α-L-rhamnosidase and β-glucosidase [34].
Conclusion
Our study evaluated chocolate as a potential oral delivery system of naringin. Ethanol was chosen as to extract naringin from grapefruit peel due to its volatility and lesser toxicity when compared to methanol. A simple, rapid, accurate and precise HPLC-DAD was developed to quantify naringin in the fortified chocolate. While increase in bitterness was found to be appealing to some, further studies may seek to minimize organoleptic changes through the addition of sweeteners, nanoencapsulation, or the enzymatic hydrolysis of naringin.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Srinivasan S, Vinothkumar V, Murali R, Watson RR (2019) Antidiabetic efficacy of citrus fruits with special allusion to flavone glycosides. In: Watson RR, Preedy VR (eds) Bioactive food as dietary interventions for diabetes, 2nd edn. Academic Press, Massachusetts, pp 335–346
Ahmed S, Khan H, Aschner M, Hasan MM, Hassan STS (2019) Therapeutic potential of naringin in neurological disorders. Food Chem Toxicol 132:110646. https://doi.org/10.1016/j.fct.2019.110646
Moghaddam HR, Samimi Z, Moradi SZ, Little PJ, Xu S, Farzaei MH (2020) Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur J Pharmacol 887:173535. https://doi.org/10.1016/j.ejphar.2020.173535
Yang Y, Trevethan M, Wang S, Zhao L (2022) Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: an update on bioavailability, pharmacokinetics, and mechanisms. J Clin Biochem Nutr 104:108967. https://doi.org/10.1016/j.jnutbio.2022.108967
Elsawy H, Alzahrani AM, Alfwuaires M, Abdel-Moneim AM, Khalil M (2021) Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed Pharmacother 143:112180. https://doi.org/10.1016/j.biopha.2021.112180
Chtourou Y, Aouey B, Aroui S, Kebieche M, Fetoui H (2016) Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem Biol Interact 243:1–9. https://doi.org/10.1016/j.cbi.2015.11.019
Adetunji JA, Fasae KD, Awe AI, Paimo OK, Adegoke AM, Akintunde JK, Sekhoacha MP (2023) The protective roles of citrus flavonoids, naringenin, and naringin on endothelial cell dysfunction in diseases. Heliyon 9:e17166. https://doi.org/10.1016/j.heliyon.2023.e17166
Memariani Z, Abbas SQ, ul Hassan SS, Ahmadi A, Chabra A (2021) Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol Res 171:105264. https://doi.org/10.1016/j.phrs.2020.105264
Sharma A, Bhardwaj P, Arya SK (2021) Naringin: a potential natural product in the field of biomedical applications. Carbohydr Polym Techn Appl 2:100068. https://doi.org/10.1016/j.carpta.2021.100068
Li P, Wu H, Wang Y, Peng W, Su W (2020) Toxicological evaluation of naringin: acute, subchronic, and chronic toxicity in beagle dogs. Regul Toxicol Pharmacol 111:104580. https://doi.org/10.1016/j.yrtph.2020.104580
Say A, Ayar A, Çakir D (2017) Interaction between grapefruit juice and drugs. Acta Phys Pol 132:1030–1031. https://doi.org/10.12693/aphyspola.132.1030
Ukom AN, Ezenwigbo MC, Ugwuona FU (2022) Grapefruit peel powder as a functional ingredient in cake production: effect on the physicochemical properties, antioxidant activity and sensory acceptability of cakes during storage. Int J Gastron Food Sci 28:100517. https://doi.org/10.1016/j.ijgfs.2022.100517
Hassam MAM, Hussein SM (2018) Chemical and technological studies on pink grapefruit (Citrus paradise L.) peels. 2- physicochemical properties and technological quality of cake fortified with different levels of pink grapefruit (Citrus paradise L.) peels powder. World J Dairy Food Sci 13:1–8. https://doi.org/10.5829/idosi.wjdfs.2018.01.08
Teng J, Liu X, Hu X, Zhao Y, Tao N, Wang M (2018) Dihydromyricetin as a functional additive to enhance antioxidant capacity and inhibit the formation of thermally induced food toxicants in a cookie model. Heliyon 23:2184. https://doi.org/10.3390/molecules23092184
Zhang X, Chen F, Wang M (2014) Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J Agric Food Chem 62:1643–1648. https://doi.org/10.1021/jf4045827
Nishad J, Dutta A, Saha S, Rudra SG, Varghese E, Sharma RR, Tomar M, Kumar M, Kaur C (2021) Ultrasound-assisted development of stable grapefruit peel polyphenolic nano-emulsion: optimization and application in improving oxidative stability of mustard oil. Food Chem 334:127561. https://doi.org/10.1016/j.foodchem.2020.127561
Ali SM, Imran A, Arshad MU, Ahmed RS, Imran M (2021) Physicochemical, antioxidant and enzymes activities of grape fruit peel and pomace enriched functional drinks. Cell Mol Biol 67:125–131. https://doi.org/10.14715/cmb/2021.67.1.19
Wedamulla NE, Fan M, Choi Y-J, Kim EK (2022) Citrus peel as a renewable bioresource: transforming waste to food additives. J Funct Foods 95:105163. https://doi.org/10.1016/j.jff.2022.105163
Kaur S, Panesar PS, Chopra HK (2021) Citrus processing by-products: an overlooked repository of bioactive compounds. Crit Rev Food Sci Nutr 1–20. https://doi.org/10.1080/10408398.2021.1943647
Martini S, Conte A, Tagliazucchi D (2018) Comprehensive evaluation of phenolic profile in dark chocolate and dark chocolate enriched with sakura green tea leaves or turmeric powder. Food Res Int 112:1–16. https://doi.org/10.1016/j.foodres.2018.06.020
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A (2013) Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 14:1818–1892. https://doi.org/10.1089/ars.2012.4581
Feng C, García-Martín JF, Broncano Lavado M, López‐Barrera M, del Álvarez‐Mateos C P (2020) Evaluation of different solvents on flavonoids extraction efficiency from sweet oranges and ripe and immature seville oranges. Int J Food Sci 55:3123–3134. https://doi.org/10.1111/ijfs.14576
Wang Z, Shang Q, Wang W, Feng X (2009) Microwave-assisted extraction and liquid chromatography/mass spectrometry analysis of flavonoids from grapefruit peel. J Food Process Eng 34:844–859. https://doi.org/10.1111/j.1745-4530.2009.00513.x
Li P, Yao X, Zhou Q, Meng X, Zhou T, Gu Q (2022) Citrus peel flavonoid extracts: health-beneficial bioactivities and regulation of intestinal microecology in vitro. Front Nutr 9:1–13. https://doi.org/10.3389/fnut.2022.888745
Li P, Wang S, Guan X, Cen X, Hu C, Peng W, Wang Y, Su W (2014) Six months chronic toxicological evaluation of naringin in sprague–dawley rats. Food Chem Toxicol 66:65–75. https://doi.org/10.1016/j.fct.2014.01.023
Sik B, Lakatos EH, Kapcsándi V, Székelyhidi R, Ajtony Z (2021) Exploring the rosmarinic acid profile of dark chocolate fortified with freeze-dried lemon balm extract using conventional and non-conventional extraction techniques. LWT-Food Sci Technol 147:111520. https://doi.org/10.1016/j.lwt.2021.111520
Belščak-Cvitanović A, Komes D, Durgo K, Vojvodić A, Bušić A (2015) Nettle (Urtica dioica L.) extracts as functional ingredients for production of chocolates with improved bioactive composition and sensory properties. J Food Sci Technol 52:7723–7734. https://doi.org/10.1007/s13197-015-1916-y
Poliński S, Topka P, Tańska M, Kowalska S, Czaplicki S, Szydłowska-Czerniak A (2022) Impact of bioactive compounds of plant leaf powders in white chocolate production: changes in antioxidant properties during the technological processes. Antioxidants 11:752. https://doi.org/10.3390/antiox11040752
Carvalho JCS, Romoff P, da Silva Lannes SC (2018) Improvement of nutritional and physicochemical proprieties of milk chocolates enriched with kale (Brassica olereacea var. Acephala) and grape (Vitis vinífera). Food Sci Technol 38:551–560. https://doi.org/10.1590/fst.15018
Kaur M, Kumar S, Bhat ZF, Bekhit AEDA, Bhatti MA (2021) Development of composite meat chocolate fortified with calcium and plant extracts. Food Biosci 42:101082. https://doi.org/10.1016/j.fbio.2021.101082
Poliński S, Kowalska S, Topka P, Szydłowska-Czerniak A (2021) Physicochemical, antioxidant, microstructural properties and bioaccessibility of dark chocolate with plant extracts. Molecules 26:5523. https://doi.org/10.3390/molecules26185523
Caballero S, Li YO, McClements DJ, Davidov-Pardo G (2021) Encapsulation and delivery of bioactive citrus pomace polyphenols: a review. Crit Rev Food Sci Nutr 62:1–17. https://doi.org/10.1080/10408398.2021.1922873
Binello A, Robaldo B, Barge A, Cavalli R, Cravotto G (2007) Synthesis of cyclodextrin-based polymers and their use as debittering agents. J Appl Polym 107:2549–2557. https://doi.org/10.1002/app.27249
Ni H, Zhang SF, Gao QF, Hu Y, Jiang ZD, Chen F (2015) Development and evaluation of simultaneous quantification of naringin, prunin, naringenin, and limonin in citrus juice. Food Sci Biotechnol 24:1239–1247. https://doi.org/10.1007/s10068-015-0159-z
Funding
Open access funding provided by Széchenyi István University (SZE). Supported by the ÚNKP-22-1-I-SZE-25 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Author information
Authors and Affiliations
Contributions
Zsolt Ajtony: Methodology, Investigation, Supervision, Conceptualization, Visualization. Beatrix Sik: Methodology, Correction, Supervision, Visualization. Aron Csuti: Investigation, Analysis, Writing, Visualization, Literature review.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no known conflicting financial or relational interest that may have influenced the contents or methodology within this work.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ajtony, Z., Sik, B. & Csuti, A. Examining the Naringin Content and Sensory Characteristics of Functional Chocolate Fortified with Grapefruit Peel Extract. Plant Foods Hum Nutr 78, 533–538 (2023). https://doi.org/10.1007/s11130-023-01091-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01091-5