Abstract
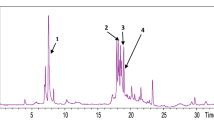
This study aimed at evaluating and comparing the chemical profile obtained by HPLC-ESI-MSn analysis, the inhibitory activity of enzymes linked to obesity (α-amylase, α-glucosidase, and lipase) and the antioxidant properties (DPPH, ABTS, FRAP, and β-carotene bleaching tests) of ethanol extracts of bulbs (BE) and aerial parts (APE) from Allium commutatum Guss. (known in Italy as “aglio delle isole”). The chemical profile revealed alliin as the main abundant compound with values of 31.5 and 38.8 mg/g extract for BE and APE, respectively. APE is rich also in quercetin (38.5 mg/g extract) and luteolin (31.8 mg/g extract). Bulbs extract exhibited the highest activity as inhibitor of enzymes linked to obesity. Except for DPPH test, APE showed the highest antioxidant potential with IC50 of 7.6 and 56.6 μg/mL in ABTS and β-carotene bleaching test after 60 min of incubation, respectively. In conclusion, the present investigation revealed A. commutatum bulbs and aerial parts as a promising source of inhibitors of enzyme linked to the obesity and of antioxidant compounds.

Similar content being viewed by others
Abbreviations
- APE:
-
Aerial Parts Extract
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- BE:
-
Bulbs Extract
- BHT:
-
Butylated Hydroxytoluene
- DM2:
-
Diabetes Mellitus Type 2
- DPPH:
-
2,2-Diphenyl-1Picrylhydrazyl
- FRAP:
-
Ferric Reducing Ability Power
- GAS:
-
Global Antioxidant Score
- HPLC-ESI-MSn :
-
High-Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry
- IC50 :
-
Half Maximal Inhibitory Concentration
- ROS:
-
Reactive Oxygen Species
- SD:
-
Standard Deviation
References
Kamenetsky R, Rabinowitch HD (2006) The genus Allium: a developmental and horticultural analysis. Hortic Rev 32:329–378. https://doi.org/10.1002/9780470767986.ch7
Gyawali R, Ibrahim SA (2014) Natural products as antimicrobial agents. Food Control 46:412–429. https://doi.org/10.1016/j.foodcont.2014.05.047
Sharifi-Rad J, Mnayer D, Tabanelli G, Stojanović-Radić ZZ, Sharifi-Rad M, Yousaf Z, Vallone L, Setzer WN, Iriti M (2016) Plants of the genus Allium as antibacterial agents: from tradition to pharmacy. Cell Mol Biol (Noisy-le-grand) 62:57–68
Martins N, Petropoulos S, Ferreira IC (2016) Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: a review. Food Chem 211:41–50. https://doi.org/10.1016/j.foodchem.2016.05.029
Hosseini A, Hosseinzadeh H (2015) A review on the effects of Allium sativum (garlic) in metabolic syndrome. J Endocrinol Investig 38:1147–1157. https://doi.org/10.1007/s40618-015-0313-8
Besendorfer V, Samardžija M, Zoldoš V, Šolić ME, Papeš D (2002) Chromosomal organization of ribosomal genes and NOR-associated heterochromatin, and NOR activity in some populations of Allium commutatum Guss. (Alliaceae). Botanical J 139:99–108. https://doi.org/10.1046/j.1095-8339.2002.00047.x
Unuofin JO, Otunola GA, Afolayan AJ (2018) In vitroα-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.). Cogn Heliyon 4:e00810. https://doi.org/10.1016/j.heliyon.2018.e00810
Golay A, Ybarra J (2005) Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab 9:649–663. https://doi.org/10.1016/j.beem.2005.07.010
Kaneto H, Katakami N, Matsuhisa M, Matsuoka T (2010) Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Med Inflam article ID 453892, 1–11. https://doi.org/10.1155/2010/453892
Garza AL, Milagro FI, Boque N, Campion J, Martínez JA (2011) Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med 77:773–785. https://doi.org/10.1055/s-0030-1270924
Tundis R, Loizzo MR, Menichini F (2010) Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini-Rev Med Chem 10:315–331. https://doi.org/10.2174/138955710791331007
Tundis R, Loizzo MR, Bonesi M, Sicari V, Ursino C, Manfredi I, Conidi C, Figoli A, Cassano A (2018) Concentration of bioactive compounds from elderberry (Sambucus nigra L.) juice by nanofiltration membranes. Plant Foods Hum Nutr 73:336–343. https://doi.org/10.1007/s11130-018-0686-x
El-shiekh RA, Al-Mahdy DA, Hifnawy MS, Abdel-Sattar EA (2019) In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. South Afr J Bot 123:43–50. https://doi.org/10.1016/j.sajb.2019.01.022
Loizzo MR, Lucci P, Núñez O, Tundis R, Balzano M, Frega NG, Conte L, Moret S, Filatova D, Moyano E, Pacetti D (2019) Native colombian fruits and their by-products: phenolic profile, antioxidant activity and hypoglycaemic potential. Foods 8:89–99. https://doi.org/10.3390/foods8030089
Todorovic V, Milenkovic M, Vidovic B, Todorovic Z, Sobajic S (2017) Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci 82:1020–1027. https://doi.org/10.1111/1750-3841.13672
Liguori L, Califano R, Albanese D, Raimo F, Crescitelli A, Di Matteo M (2017). Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. J Food Qual, 2017, Article ID 6873651:1–9. https://doi.org/10.1155/2017/6873651
Kim S, Kima DB, Jina W, Parka J, Yoona W, Leea Y, Kima S, Leea S, Kima S, Leeb OH, Shina D, Yooa M (2018) Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat Prod Res 32:1193–1197. https://doi.org/10.1080/14786419.2017.1323211
Ademoyegun OT, Adewuyi GO, Fariyike TA (2010) Effect of heat treatment on antioxidant activity of some spices. Cont J Food Sci Technol 4:53–59
Riggi E, Avola G, Siracusa L, Ruberto G (2013) Flavonol content and biometrical traits as a tool for the characterization of "Cipolla di Giarratana": a traditional Sicilian onion landrace. Food Chem 140:810–816. https://doi.org/10.1016/j.foodchem.2012.10.134
Tedesco I, Carbone V, Spagnuolo C, Minasi P, Russo GL (2015) Identification and quantification of flavonoids from two southern italian cultivars of Allium cepa L., Tropea (red onion) and Montoro (copper onion), and their capacity to protect human erythrocytes from oxidative stress. J Agric Food Chem 63:5229–5238. https://doi.org/10.1021/acs.jafc.5b01206
Figueiredo-González M, Martínez-Carballo E, Cancho-Grande B, Santiago JL, Martínez MC, Simal-Gándara J (2012) Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. J Agric Food Chem 130:9–19. https://doi.org/10.1016/j.foodchem.2011.06.006
Vukics V, Guttman A (2010) Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom Rev 29:1–16. https://doi.org/10.1002/mas.20212
Fabre N, Rustan I, de Hoffmann E, Quetin-Leclercq J (2001) Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J Am Soc Mass Spectrom 12:707–715. https://doi.org/10.1016/S1044-0305(01)00226-4
Oboh G, Ademiluyi AO, Agunloye OM, Ademosun AO, Ogunsakin BG (2019) Inhibitory effect of garlic, purple onion, and white onion on key enzymes linked with type 2 diabetes and hypertension. J Diet Suppl 16:105–118. https://doi.org/10.1080/19390211.2018.1438553
Safaeian L, Zolfaghari B, Karimi S, Talebi A, Ghazvini MA (2018) The effects of hydroalcoholic extract of Allium elburzense Wendelbo bulb on dexamethasone-induced dyslipidemia, hyperglycemia, and oxidative stress in rats. Res Pharm Sci 13:22–29. https://doi.org/10.4103/1735-5362.220964
Zhai B, Zhang C, Sheng Y, Zhao C, He X, Xu W, Huang K, Luo Y (2018) Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci Rep 8:3527. https://doi.org/10.1038/s41598-018-21421-x
Štajner D, Igić R, Popović BM, Malenčić D (2008) Comparative study of antioxidant properties of wild growing and cultivated Allium species. Phytother Res 22:113–117. https://doi.org/10.1002/ptr.2278
Suleria HAR, Butt MS, Anjum FM, Saeed F, Batool R, Ahmad AN (2012) Aqueous garlic extract and its phytochemical profile; special reference to antioxidant status. Int J Food Sci Nutr 63:431–439. https://doi.org/10.3109/09637486.2011.634786
Jalalvand AR, Zhaleh M, Goorani S, Zangeneh MM, Seydi N, Zangeneh A, Moradi R (2019) Chemical characterization and antioxidant, cytotoxic, antibacterial, and antifungal properties of ethanolic extract of Allium saralicum RM Fritsch leaves rich in linolenic acid, methyl ester. J Photochem Photobiol B 192:103–112. https://doi.org/10.1016/j.jphotobiol.2019.01.017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loizzo, M.R., Tundis, R., Sut, S. et al. High-Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry (HPLC-ESI-MSn) Analysis and Bioactivity Useful for Prevention of “Diabesity” of Allium commutatum Guss. Plant Foods Hum Nutr 75, 124–130 (2020). https://doi.org/10.1007/s11130-019-00782-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-019-00782-2