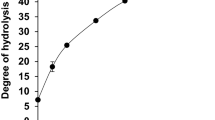
Abstract
Ingestion of diets with antithrombotic and antioxidant components offer a convenient and effective way to prevent and reduce the incidence of cardiovascular diseases. The aim of the present work was to obtain an amaranth hydrolysate by the activation of an endogenous aspartic protease, to establish adequate experimental conditions, and to evaluate its antithrombotic and antioxidant activity in order to assess its potential application as an ingredient in functional foods. The results obtained not only confirmed the presence of an endogenous protease in the amaranth isolate, but also allowed us to select an adequate incubation conditions (pH 2, 40 °C, 16 h). The hydrolysate obtained (degree of hydrolysis 5.3 ± 0.4 %) showed potential antithrombotic activity (IC50 = 5.9 ± 0.1 mg soluble protein/mL) and had more antioxidant activity than the isolate, indicating that the activation of the protease released bioactive peptides from amaranth proteins. Decreasing the pH is a simple and cheap process and is another way to obtain potential functional ingredients with bioactive compounds.



Similar content being viewed by others
Abbreviations
- DH%:
-
Degree of hydrolysis
- IC50 :
-
Concentration of isolate or hydrolysate that inhibits 50 % of the thrombus formation or produces 50 % radical neutralization
- HEP :
-
Hydrolysate prepared by activation of endogenous protease
References
Hasler CM, Bloch AS, Thomson CA, Enrione E, Manning C (2004) Position of the American Dietetic Association: functional foods. J Am Diet Assoc 104:814–826
Martínez-Maqueda D, Miralles B, Recio I, Hernández-Ledesma B (2012) Antihypertensive peptides from food proteins: a review. Food Funct 3:350–361
Madureira AR, Tavares T, Gomes AMP, Pintado ME, Malcata FX (2010) Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci 93:437–455
Kim SK, Wijesekara I (2010) Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods 2:1–9
Udenigwe CC, Aluko RE (2012) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77:11–24
Silva-Sánchez C, Barba de la Rosa AP, León-Galván MF, De Lumen BO, De León-Rodríguez A, González de Mejía E (2008) Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J Agric Food Chem 56:1233–1240
López VRL, Razzeto GS, Escudero NL, Giménez MS (2013) Biochemical and molecular study of the influence of Amaranthus hypochondriacus flour on serum and liver lipids in rats treated with ethanol. Plant Foods Hum Nutr 68:396–402
Tironi V, Añón MC (2010) Amaranth as a source of antioxidant peptides: effect of proteolysis. Food Res Int 43:315–322
Sabbione AC, Scilingo A, Añón MC (2015) Potential antithrombotic activity detected in amaranth proteins and its hydrolysates. LWT-Food Sci Technol 60:171–177
Ventureira JL, Martínez EN, Añón MC (2012) Effect of acid treatment on structural and foaming properties of soy amaranth protein mixtures. Food Hydrocoll 29:272–279
Hemalatha KPJ, Siva Prasad D (2003) Changes in the metabolism of protein during germination of sesame (Sesamum indicum L.) seeds. Plant Foods Hum Nutr 58:1–10
Schaller A (2004) A cut above the rest: the regulatory function of plant proteases. Planta 220:183–197
Martínez EN, Añón MC (1996) Composition and structural characterization of amaranth proteins isolates. An electrophoretic and calorimetric study. J Agric Food Chem 44:2523–2530
Paredes-López O (1994) Amaranth: biology, chemistry and technology. CRC Press, Boca Raton, FL
Martínez EN, Castellani OF, Añón MC (1997) Common molecular features among amaranth storage proteins. J Agric Food Chem 45:3832–3839
Schägger H (2006) Tricine-SDS-PAGE. Nat Protoc 1:16–22
Nielsen PM, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66:642–646
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Yang WG, Wang Z, Xu SY (2007) A new method for determination of antithrombotic activity of egg white protein hydrolysate by microplate reader. Chin Chem Lett 18:449–451
Zhang S, Wang Z, Xu SY (2008) Antioxidant and antithrombotic activities of rapeseed peptides. JAOCS 85:521–527
Orsini Delgado MC, Galleano M, Añón MC, Tironi VA (2015) Amaranth peptides from simulated gastrointestinal digestion: antioxidant activity against reactive species. Plant Foods Hum Nutr 70:27–34
Siddhuraju P (2006) The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (jacq.) marechal seed extracts. Food Chem 99:149–157
Atanassov A, Tchorbanov B (2009) Synthetic and natural peptides as antithrombotic agents. A view on the current development. Biotechnol Biotechnol Equip 23:1109–1114
Abugoch LE, Martínez EN, Añón MC (2010) Influence of pH on structure and function of amaranth (A. hypochondriacus) protein isolates. Cereal Chem 87:448–453
Walsh R, Martin E, Darvesh S (2010) A method to describe enzyme-catalyzed reactions by combining steady state and time course enzyme kinetic parameters. Biochim Biophys Acta 1800:1–5
Condés MC, Scilingo A, Añón MC (2009) Characterization of amaranth proteins modified by trypsin proteolysis. Structural and functional changes LWT 42:963–970
Marcone MF, Kakuda Y (1999) A comparative study of functional properties of amaranth and soybean globulins isolates. Nahrung 43:368–373
Quiroga AV, Martínez EN, Rogniaux H, Geairon A, Añón MC (2010) Amaranth (Amaranthus hypochondriacus) vicilin subunit structure. J Agric Food Chem 58:12957–12963
Laudano AP, Doolittle RF (1978) Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci USA 75:3085–3089
Lee KA, Kim SH (2005) SSGE and DEE, new peptides isolated from a soy protein hydrolysate that inhibit platelet aggregation. Food Chem 90:389–394
Hyun KW, Jeong SC, Lee DH, Park JS, Lee JS (2006) Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 27:1173–1178
Jo HY, Jung WK, Kim SK (2008) Purification and characterization of a novel anticoagulant peptide from marine echiuroid worm Urechis unicinctus. Process Biochem 43:179–184
Gan ZR, Gould RJ, Jacobs JW, Freidman PA, Polokoff MA (1988) Echistatin, a potent platelet aggregation inhibitor from venom of viper, Echis carinatus. J Biol Chem 263:19827–19832
Re R, Pellegrini A, Proteggente A, Pannala M, Yang C, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Orsini Delgado MC, Tironi VA, Añón MC (2011) Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT 44:1752–1760
Acknowledgments
This work was supported by PIP - CONICET 11220110101109.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors, A.C. Sabbione, S.M. Ibañez, E.N. Martínez, M.C. Añón, and A. Scilingo, declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sabbione, A.C., Ibañez, S.M., Martínez, E.N. et al. Antithrombotic and Antioxidant Activity of Amaranth Hydrolysate Obtained by Activation of an Endogenous Protease. Plant Foods Hum Nutr 71, 174–182 (2016). https://doi.org/10.1007/s11130-016-0540-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-016-0540-y