Abstract
Accurate estimation of photosynthetic parameters is essential for understanding plant physiological limitations and responses to environmental factors from the leaf to the global scale. Gas exchange is a useful tool to measure responses of net CO2 assimilation (A) to internal CO2 concentration (Ci), a necessary step in estimating photosynthetic parameters including the maximum rate of carboxylation (Vcmax) and the electron transport rate (Jmax). However, species and environmental conditions of low stomatal conductance (gsw) reduce the signal-to-noise ratio of gas exchange, challenging estimations of Ci. Previous works showed that not considering cuticular conductance to water (gcw) can lead to significant errors in estimating Ci, because it has a different effect on total conductance to CO2 (gtc) than does gsw. Here we present a systematic assessment of the need for incorporating gcw into Ci estimates. In this study we modeled the effect of gcw and of instrumental noise and quantified these effects on photosynthetic parameters in the cases of four species with varying gsw and gcw, measured using steady-state and constant ramping techniques, like the rapid A/Ci response method. We show that not accounting for gcw quantitatively influences Ci and the resulting Vcmax and Jmax, particularly when gcw exceeds 7% of the total conductance to water. The influence of gcw was not limited to low gsw species, highlighting the importance of species-specific knowledge before assessing A/Ci curves. Furthermore, at low gsw instrumental noise can affect Ci estimation, but the effect of instrumental noise can be minimized using constant-ramping rather than steady-state techniques. By incorporating these considerations, more precise measurements and interpretations of photosynthetic parameters can be obtained in a broader range of species and environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gas exchange is a powerful tool in plant physiology that allows us to understand CO2 and water relations in plants to compare plant performance in a range of environments (Stinziano and Way 2017; Vincent et al. 2017; Smith et al. 2020), to describe how plants cope with challenging environments (Kumarathunge et al. 2019; Zhu et al. 2020), and to model plant-environment interactions up to the global scale (Oleson et al. 2013; Rogers et al. 2017; Lombardozzi et al. 2018). However, gas exchange measurements require a sufficient signal-to-noise ratio to obtain high-quality data for inferences. This can be achieved by ensuring stomata are adequately open or by incorporating a model that accounts for cuticular conductance (gcw) (Márquez et al. 2021). However, many lines of research specifically address either species or conditions that produce low stomatal conductance, including xerophytic species, conditions of water deficit, or high temperatures. A comprehensive understanding of the factors affecting gas exchange measurements, including stomatal conductance (gsw) and gcw, is crucial for obtaining accurate data and drawing sound conclusions (See Table 1 for a list of abbreviations and equations).
Stomatal conductance is a vital process that has a direct impact on plant growth and productivity, as it constrains photosynthesis and water-use. Plant species present a wide variation in gsw depending on leaf morphology, physiology, ecotype, and environment, with many exhibiting low conductance. Low gsw presents a challenge to the estimation of photosynthesis, particularly in C3 species with maxima below 0.2 mol m−2 s−1 (Körner et al. 1986; Tezara et al. 1998), and rapid stomatal closure whenever conditions are not ideal (Radin et al. 1994) or at a particular time of day (Steppe et al. 2006). For example, in a survey of Citrus x sinensis (L.) and C. reticulata (Blanco), 35% of the mid-morning (9:00 am-11:00 am) gas exchange measurements exhibited total leaf conductance to water (glw) below 0.08 mol m−2 s−1 (Vincent et al., unpublished data). When glw is low, the signal-to-noise ratio in gas exchange measurements is also low. Additionally, it becomes difficult to obtain a sufficient range in intercellular CO2 concentration (Ci) to characterize the response of net CO2 assimilation (A) to Ci (A/Ci curves) (Stinziano et al. 2020). Further compounding the signal-to-noise challenge in the context of A/Ci curves, plants respond to elevated CO2 by stomatal closure on an order of minutes.
Using A/Ci curves, we can apply the Farquhar-von Caemmerer-Berry model of photosynthesis (Farquhar et al. 1980) to estimate photosynthetic parameters for comparing plant performance (Way and Sage 2008), understanding photosynthetic acclimation (Kumarathunge et al. 2019), and modelling vegetative carbon uptake (Oleson et al. 2013). In species or conditions with low gsw or stomata that close rapidly at high CO2, such as those seen in evergreen broadleaved species, these responses are difficult to characterize (Lin et al. 2023). Stomatal limitations may further lead to incorrect conclusions on what is limiting photosynthesis. For example, it is possible to misinterpret a stomatal limitation as a constraint related to Rubisco carboxylation. The recent development of rapid A/Ci response (RACiR) curves (Stinziano et al. 2017, 2019a, b) and subsequently of the dynamic acclimation approach which allows more efficient use of constant-ramping methods (Saathoff and Welles 2021) may be sufficiently fast to circumvent the difficulties of stomatal closure in such species.
Although glw is often assumed equal to gsw, this is not entirely accurate. Cuticular conductance (gcw) is a component of glw and a key factor that impacts gas exchange measurements in plants, as it involves the diffusion of gases across the cuticle layer of leaves (Márquez et al. 2022). Although in practice it is usually not considered, gcw can impact A/Ci measurement, because the ratio of conductance to H2O and to CO2 is dramatically different between stomata and cuticles. The diffusivity of H2O and CO2 through the stomata have a ratio of 1.6, which forms the basis of estimates of stomatal conductance to CO2. However, the cuticle is a stronger barrier to CO2 than H2O where the diffusivity of H2O is much higher than that of CO2 with a ratio of 1:20–40 (Boyer et al. 1997; Boyer 2015b). In addition, overlooking gcw can be problematic because, in certain cases, it can reach levels as high as 28% of glw (Holmgren et al. 1965; Boyer et al. 1997). Because estimates of Ci are based on total conductance to CO2 (gtc), assuming that glw = gsw can lead to misestimating Ci (Table 1; Boyer 2015b). Variations in gcw among plant species can significantly affect the accuracy of gas exchange measurements (Márquez et al. 2021; Boyer et al. 1997), particularly in low-conductance species (Márquez et al. 2022).
Previous studies have highlighted the impact of gcw on gas exchange measurements and underscored the need for appropriate correction methods to ensure accurate measurements (Grassi and Magnani 2005; Tominaga et al. 2018). However, estimating gcw separately from gsw, which usually governs gas diffusion through the leaf surface under illuminated conditions, has been a major challenge. Consequently, it is often neglected in gas exchange calculations. In this regard, a recent study by Márquez et al. (2022) has proposed a new approach, called the red-light method, which estimates gcw from gas exchange measurements and a known CO2 concentration within the leaf during photosynthetic induction under red light. This novel method enables accurate estimation of gcw, the inclusion of which can improve the precision of gas exchange measurements. Lamour et al. (2022) recently provided a theoretical reassessment of the impact of gcw in the measurement of A/Ci curves and the parameters calculated from them, using real A/Ci curves and applying a range of hypothetical gcw. In practice, many researchers face species or conditions of highly variable glw. Thus, knowledge of the impact of these factors and how to circumvent the resulting limitations in practice is essential, especially under low glw conditions.
In this work, our objective was to assess the impact of gcw, gsw, and other sources of error on the validity of estimates of photosynthetic parameters. To test the impacts of gcw and gsw we selected four species that vary in both. We used the red-light method of gcw measurement and steady state and RACiR methods to estimate A/Ci-based parameters in these species with varying gcw and gsw. Although the RACiR method has been superseded by a dynamic assimilation technique (DAT; Tejera-Nieves et al. 2024), which eliminated the need for running empty-chamber curves, the utility of both RACiR and DAT in terms of the use of constant-ramping and frequent logging to assess A/Ci are interchangeable. We modeled the impact of glw and gcw on Ci estimates and developed criteria to understand the impact of conductance on the reliability of A/Ci curves and the resulting parameters and provide this model for future work.
Materials and methods
Modeling the impact of stomatal and cuticular conductance on estimates of internal CO 2
To estimate the impact of glw on the estimation of Ci we modeled the estimation of Ci in response to varying external CO2 concentrations (Ca) and gsw. To achieve this we used the Photosyn() command in the {plantecophys} package (Duursma 2015). This model allowed us to fix vapor pressure deficit (1.5 kPa) and Ca (100, 400, or 2000 ppm), along with temperature (25 °C) and maximum rates of carboxylation (50 µmol m−2 s−1) and electron transport (100 µmol m−2 s−1), which are the default values in the model. We considered a range of glw values from 0.001 to 1 mol m−2 s−1 in increments of 0.001 mol m−2 s−1 to cover the full range of expected values from medium to low conductance species. Ci was initially calculated according to the standard calculations of most gas exchange equipment. This calculation is based on von Caemmerer and Farquhar (1981) as:
where gtc is total conductance to CO2, which was estimated as:
where 1.6 and 1.37 are the ratios of diffusivity of CO2 to H2O in air (in stomata) and in the boundary layer. K is the ratio of stomatal resistance of the adaxial to abaxial sides of the leaf. For this consideration, we set K = 0, which is common for low conductance species, and boundary layer conductance to water (gbw) as 2.23 mol m−2 s−1. These equations are standard for estimates in gas exchange. If K = 0 and gbw is large enough to be ignored, as is expected in most gas exchange measurement contexts (Márquez et al. 2021), then Eq. 2 can be simplified as: \({g}_{tc}=\frac{{g}_{sw}}{1.6}\), which assumes that glw≈gsw.
At high glw, this assumption may be roughly correct. However, as glw decreases relative to gcw, the assumption is likely to become less tenable and increasingly impact estimates of Ci, because the cuticular conductance to CO2 (gcc) is expected to be approximately 0.05 gcw (Márquez et al. 2022). Thus, we corrected the original glc by calculating:
where 1:20 is the expected proportion of gcw to gcc (Boyer et al. 1997). Because the Photosynth() command assumes glw = gsw and does not allow explicit parameterization of gtc, but calculates gtc = glw/1.6 (Duursma 2015), we recalculated a pseudo-glw to result in the corrected gtc by multiplying the corrected gtc by 1.6. Ci was then recalculated based on the new estimate of glc. We then calculated the proportional misestimation of Ci (Ci mis) when not accounting for gcw as the difference between uncorrected Ci (Ci uncorr) and corrected Ci (Ci cor) as a proportion of Ci Ci cor:
Plant material
Papaya (Carica papaya L.), bell pepper (Capsicum annuum L.), citrus (C. x sinensis cv. ‘Valencia’) and magnolia (Magnolia grandiflora L.) plants were grown in a greenhouse in Lake Alfred, FL 33850, USA (28.1021° N, 81.7121° W) in a natural diurnal cycle and daily maximum and minimum of ~ 25–34 °C. All plants were irrigated twice a week to ensure that the growing media 'Pro-mix BX' (Premier Tech Ltd, Quebec, Canada) remained adequately moist and were fertilized with 12-4-8 Miracle-Gro liquid fertilizer (The Scotts Company, LLC) every 2 weeks. SS and RACiR curves were performed on healthy and fully expanded leaves. In total, five plants each for bell pepper, magnolia, and papaya were measured, while 18 citrus plants were used.
Gas exchange measurements
Response curves were measured using a portable infrared gas analyzer (LI-6800, Li-COR Inc., Lincoln, NE, USA) (Console version: Bluestem v.1.4.02) equipped with a 3 × 3 cm leaf chamber (9 cm2). Leaf chamber settings were as follows: fan speed of 10,000 rpm, flow rate of 300 μmol s−1 and 600 μmol s−1 (for RACiR and SS, respectively), overpressure of 0.1 kPa, 24 mmol mol−1 of H2O in the reference cell producing between ~ 63% and 79% relative humidity (RH) in the leaf chamber, and producing a variation of ~ 2 to 3% in RH over the course of measurements, photosynthetically active radiation of 1200 µmol m−2 s−1 [90% red and 10% blue light], and chamber air temperature at 25 °C. We first measured paired standard RACiR and SS to compare parameter estimates from each. Each set of RACiR and SS curves was gathered on adjacent leaves of the same plant (not on the same leaf to avoid the effects of the first curve on the results of the second) on the same day.
Rapid A/C i response (RACiR) curves
RACiR data were gathered beginning at a reference CO2 concentration of 100 µmol mol−1 with a ramping rate of 100 µmol mol−1 min−1, ending at 900 µmol mol−1. After testing paired measurements, we gathered five additional RACiR curves from five plants each for all four species, this time the Ca range was 100 µmol mol−1 to 2000 µmol mol−1 with the same 100 µmol mol−1 min−1 ramping rate. Before each curve, the IRGA was matched to allow the system to stabilize in match mode. The empty chamber curves were determined in exactly the same way as the RACiR curves, except that the leaves were not clamped. Moreover, one empty chamber curve was used to calibrate the two leaf runs taken within 1 h. For all measurements, the leaf chamber was clamped on the leaf and allowed to acclimate to set environmental conditions until A reached a steady state before running the CO2 response curves; however, the acclimation time was variable among species. The ‘Autolog’ program of LI-6800 was used to record data every 2 s (9 min and 19 min for RACiR when CO2 concentration was set at 100–900 µmol mol−1 and 100–2000 µmol mol−1, respectively).
Steady-state A/C i curves
The LI-6800 CO2 response program was used for the steady-state (SS) A/Ci curves. Firstly, all environmental conditions were set the same as the RACiR curves except for the CO2. Secondly, the sample CO2 was set to 380 µmol mol−1 CO2. All leaves were acclimated to set environmental conditions until A reached a steady state before running the CO2 response curves. SS curves were collected starting at a reference CO2 concentration of 380 µmol mol−1, then proceeding through the following progression conditions: 285, 190, 145, 100, 50, 380, 475, 570, 665, 760, 960, 1160, 1460, 1760, 1960 µmol mol−1 CO2 with minimum and maximum waiting times of 60 s and 120 s, respectively. The reference and sample IRGAs were matched before each measurement (Stinziano et al. 2017), and the measuring time for each SS A/Ci curve was approximately 30 min.
Measurement of cuticular conductance (g cw )
The gcw was estimated using the red-light technique introduced by Márquez et al. (2021). Briefly, the plants were dark-adapted for 12 h. A leaf was then placed in the darkened LI-6800 chamber and allowed to acclimate to 400 µmol mol−1 CO2, 25 °C, and 24 mmol mol−1 of H2O in the reference before recording dark respiration (Rdark). The dark-acclimated leaf was subsequently exposed to the same environmental conditions as those for Rdark measurements, except that 100 µmol m−2 s−1 red light was applied, and measurements were taken every 7 s during the light induction until the maximum A was achieved, and over 5 min at steady state.
To determine the rate of electron transport or the electron transport rate at a given CO2 concentration (Ja) and gamma star (Γ*, represents the CO2 photo-compensation point) during the experiment, a RACiR curve was performed under the same conditions as during the red-light induction, with the exception that stomatal opening is promoted using 100 µmol m−2 s−1 red-blue (40 µmol m−2 s−1 blue), and a CO2 concentration ranging from 100 µmol mol−1 to 1400 µmol mol−1 with a 100 µmol mol−1 min−1 ramping rate. The leaf chamber was clamped onto the leaf and allowed to acclimate to the set environmental conditions until A reached a steady state before running the CO2 response curves.
Analysis
Raw data obtained for each leaf sample were filtered and corrected using the empty chamber data as per the protocol for RACiR curve correction provided by Coursolle et al. (2019). RACiR data were calibrated using the {racir} R package (Stinziano et al. 2019a; Lawrence et al. 2019; Stinziano 2020). All curves were fit using the fitaci() command from the {plantecophys} package (Duursma 2015; Pilon et al. 2018) in R statistical software (R Core Team 2013), with options selected to fit triose phosphate utilization (TPU) limitation rate and with no temperature correction. We extracted the maximum Rubisco carboxylation capacity (Vcmax), maximum electron transport rate under saturating light (J1200), triose phosphate utilization (TPU), dark respiration (Rdark), the Ci at the CO2 to RuBP transition (Citrans1), and the Ci at RuBP to TPU transition (Citrans2) for analysis. Subsequently, all A/Ci curves were fit with uncorrected and corrected gsw values to assess the impact of gcw on parameter estimates.
Statistics
The resulting parameters from the fit curves were subjected to analysis of variance and paired Student’s t-tests by using Statistix 8.1® software to test for differences between SS and RACiR curves. These tests were performed on A/Ci curves derived with uncorrected and corrected gsw. Multiple comparisons were performed with Tukey’s honestly significant difference (Tukey’s HSD), and differences were significant when P < 0.05. Data in the figures were processed and analyzed with R (R version 4.1.1, 2021 "Kick Things").
Data and code
Data and code are available in supplementary files S1.
Results
At low g sw , C i estimates become unreasonable
There was a strong impact of glw on Ci. Ca also impacted the response dynamics, increasing the magnitude of Ci reduction in response to reduced glw (Fig. 1). The impact of glw at a Ca of 100 ppm was negligible, but its impact was strong at both 400 and 2000 ppm. This result indicated limits to the estimation of Ci at low glw, suggesting improvements in the accuracy of conductance estimates and Ci impact estimates of Ci to a greater degree at high Ca.
Ci in response to glw, where glw is assumed to equal gsw, under varying glw and scenarios of Ca = 100, 400, or 2000 µmol mol−1. Proportional Ci values are proportional to the value when gsw = 1 mol m−2 s−1. The light green ribbon shows the gap between the corrected and uncorrected Ci values, bounded on the bottom by the uncorrected Ci estimate (solid black line) and on the top by the corrected estimate (dashed black line). The correction accounts for the differential effect of gcw in the estimation of gcc and thus on Ci. The solid green line shows the proportional misestimation of Ci due to not accounting for gcw proportional to the corrected value at the same gsw. The dashed blue horizontal line denotes a proportion 0.05 (5% miscalculation or uncertainty). In all cases vapor pressure deficit was 1.5 kPa, temperature was 25 °C, maximum rate of carboxylation was 50 µmol m−2 s−1, electron transport was 100 µmol m−2 s−1, and gcw was 0.0056 mol m−2 s.−1 (results for papaya, which fell toward the middle of the distribution among the four species)
Cuticular conductance impacts estimation of internal CO 2 as a proportion of total leaf conductance
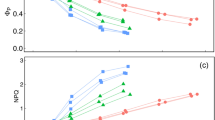
We modeled the impact of accounting for gcw for a more accurate estimate of gtc (Fig. 2), using 0.0056 mol m−2 s−1 estimate of gcw from papaya as the case, because it fell toward the middle of the range of the four species assessed in this study (See below). As glw declined, the proportional misestimation of Ci increased exponentially (Fig. 1), this can be expressed as a proportion of gcw: glw, resulting in a much greater alignment of the species tested and that the slope the response of misestimation due to the assumption that gsw = glw is much slower than in response to glw alone. These results suggest that including gcw in the calculation of Ci will be important in any context where gcw is greater than 10% of glw. Additionally, even species with relatively high glw (eg. C. annuum and C. papaya) reached sufficiently high gcw:glw to cause very large (> 50%) misestimations of Ci. The overall response of misestimation of Ci can be seen in Supplemental Fig. 1, where increasing gcw:glw increases the misestimation of Ci with moderate impacts from A, E, and Ca.
Miscalculation in estimates of leaf internal CO2 (Ci) concentrations over a range of low leaf conductance to water (glw) values considering the impact of cuticular conductance to water (gcw) in the estimation of gsw and the resulting gtc and Ci. Proportional Ci values are proportional to the value when gsw = 1 mol m−2 s−1. A shows the impact of accounting for gcw on the estimate of Ci, expressed as a proportion of the Ci estimate at glw = 1.0 mol H20 m−2 s−1. B shows the same values as a proportion of gcw to glw. The light red, green, or blue ribbons show the gap between the corrected and uncorrected Ci values, bounded by the uncorrected Ci estimate (solid black line) and the corrected estimate (dashed black line) proportional to the corrected value at the same gsw. The solid lines corresponding to the bands show the proportional misestimation of Ci due to not accounting for gcw. The horizontal error bars show ± 1 standard deviation of maximum gcw:glw in a series of five steady-state A/Ci curves for each species. Assumptions, in this case, are Vcmax = 50 µmol m−2 s−1, Ca = 400 µmol mol−1, and VPD = 1.5 kPa
Noise in the measurement of leaf conductance to water impacts estimation of internal CO 2 at low conductance
Noise is the random unexplained error associated with any measurement. Any instrument will have some noise associated with the estimation of any variable. We estimated the impact of noise on the IRGA instrument’s estimation of glw by summarizing the glw estimates across several empty-chamber rapid A/Ci curves, which had been gathered to calibrate the leaf-sample curves. Because an empty, sealed chamber with no sample is known to have a conductance of 0, we could then record the conductance to estimate the precision of the estimate of 0. The standard deviation of glw was 0.00415 mol m−2 s−1. Given that the true value of glw may easily be ± one standard deviation of the estimate at any given point, we added and subtracted this instrumental noise value from the glw estimate to provide a range of possible “true values” and re-calculated A and Ci. Instrumental noise in measuring glw greatly impacts estimates of Ci at low glw values and compounds the possible misestimation of Ci when not accounting for gcw (Fig. 3).
Uncertainty due to instrumental noise and miscalculation in estimates of leaf internal CO2 (Ci) concentrations over a range of low leaf conductance to water (glw) values considering the impact of cuticular conductance to water (gcw) and technical uncertainty in the estimation of glw. A shows Ci estimate and potential proportional miscalculation in response to glw. B shows the same variables in response to the ratio of gcw to glw. Ci values are expressed as a proportion of the Ci estimate at glw = 1.0 mol H2O m−2 s−1. Proportional error values are the difference between the uncorrected and the corrected values divided by the corrected value. Red indicates uncorrected estimates, and blue indicates corrected estimates. Dashed lines represent the estimate, while the ribbons indicate the range ± std. dev. Solid lines represent the proportional error in the Ci estimate. The purple line represents the maximum possible combined error from uncertainty in measuring glw and not accounting for gcw. This scenario considers Vcmax = 50 µmol m−2 s−1, Ca = 400 µmol mol−1, and VPD = 1.5 kPa. ambient CO2, gcw = 0.0056 µmol m−2 s−1, and the standard deviation of glw = 0.00415 mol m−2 s−1. The error in the glw is based on empty chamber measurements using an LI-6800 infrared gas analyzer (LI-COR Biosciences, Lincoln, NE). The dashed horizontal line denotes a proportion of 0.05 (5% miscalculation or uncertainty)
Considering cuticular conductance to water and instrumental noise alters A/C i response estimates
We measured the gcw of magnolia, papaya, citrus, and bell pepper, and found a wide range among these species from 0.0045 to 0.0076 mol m−2 s−1 (Table 2). Although the gcw estimates were within the range of the standard deviation of any single measurement of glw, the standard deviation of the estimates of gcw were between 0.0009 and 0.0024. The greater precision of the estimate of gcw results from the approach to measuring gcw which uses the average of glw over 5 min with measurements every 7 s Thus, each gcw measurement resulted from the mean of 42 glw measurements. We considered the gcw of each species, as well as possible instrumental noise, and applied these to the RACiR and SS A/Ci curves with the lowest observed conductance for each species (Fig. 4). The impact of accounting for gcw and instrumental noise in the estimation of glw varied by species and by the A/Ci curve method, suggesting that in many cases constant-ramping (RACiR) curves are more precise than SS curves, and that under some conditions SS curves produced estimates within 1 standard deviation of glw of unrealistic A/Ci results.
Steady-state (SS) and rapid A/Ci (RACiR) curves for bell pepper (Capsicum annuum), citrus (Citrus x sinensis), magnolia (Magnolia grandiflora), and papaya (Carica papaya). Each panel shows a single A/Ci curve. The curves with the lowest leaf conductance to water (glw) for each method for each species were selected. Red lines represent an uncorrected curve in which glw is assumed to be equivalent to stomatal conductance to water. Solid black lines show the same curve after correcting Ci estimates accounting for the species-specific cuticular conductance. Dashed lines represent the range after accounting for instrumental noise in the measurement of stomatal conductance. The standard deviation of glw = 0.00415 mol m−2 s−1, based on empty chamber measurements in an LI-6800 infrared gas analyzer (LI-COR Biosciences, Inc., Lincoln, NE, USA)
RACiR versus steady-state A/C i curves
Overall, results from SS and RACiR curves were similar (Table 3). Notable differences are the smaller Rdark estimates by SS than by RACiR and the small number of TPU estimates produced by RACiR, the latter was not surprising considering the lower pre-set range of Ca values for RACiR, which prompted us to gather a new data set to test whether consistent estimation of TPU was possible with RACiR (see below). In addition, for magnolia, Vcmax fitted to RACiR curves was lower than SS curves. In citrus, the value of gsw was higher in RACiR than in SS, illustrating that stomatal attenuation in citrus is lesser when using constant ramping than SS methods, because there is less time for stomatal responses to high Ca.
Correcting for g cw impacts the estimation of photosynthetic parameters
To illustrate the effect of the gcw on A/Ci-derived parameters, both the RACiR and SS A/Ci curves were recomputed with corrected gsw. We then estimated the photosynthetic parameters from the recalculated A/Ci data (Table 3). For magnolia and citrus, correcting for gcw affected Vcmax and J1200 estimates. In the case of SS curves, correcting for gcw affected Vcmax estimates in all species except for magnolia. With corrected gsw, no difference was found for Rdark regardless of A/Ci curve type, except for citrus, which had a greater Rdark value in RACiR with corrected gsw. Interestingly, correcting gsw decreased RACiR-derived Citrans1 for all species. However, for SS, only citrus showed a difference between corrected and uncorrected gsw-derived Citrans1 and Citrans2. The gcw correction strongly decreased gsw estimate in SS curves. However, for RACiR, only gsw estimates in magnolia were affected by gcw correction.
Stomatal limitations to gas exchange
Maximum and minimum gsw and Ci differed among species (Fig. 5). Papaya had the greatest gsw followed by bell pepper, citrus, and magnolia. The same trend was found for Ci. Each species’ gsw responded differently to changing Ca in the RACiR curves, with some exhibiting reductions in A due to low gsw. In magnolia, two curves decreased A noticeably as Ca exceeded 700 µmol mol−1 (Fig. 5). Moreover, in all the RACiR curves in magnolia, gsw decreased or remained low over time, regardless of Ci concentration. In bell pepper, gsw increased from Ca of 380 µmol mol−1 to 475 µmol mol−1, while higher Ca values decreased gsw. In citrus, gsw decreased as Ca increased above ~ 400 µmol mol−1.
Plots of net CO2 assimilation (A) and stomatal conductance (gsw) by intercellular CO2 concentration (Ci), and distributions of maximum and minimum gsw and Ci of leaves of magnolia (Magnolia grandiflora), citrus (Citrus sinensis), bell pepper (Capsicum annuum), papaya (Carica papaya) using RACiR method. P-values indicate the results of Tukey’s HSD test
RACiR curves with wide C a range
To test the range of Ci attainable using constant-ramping, we performed separate curves with elevated Ca up to 2000 µmol mol−1 (Table 4). Overall, the gsw pattern among species was the same as for previous measurements. Moreover, TPU fitted by RACiR curves was observed for all species except for one curve in magnolia (Table 4 and Fig. 4). Correcting for gcw increased the Vcmax estimate in all species, except citrus. J1200 was only impacted in magnolia. Rdark, TPU, and Citrans2 were decreased in bell pepper and papaya while Citrans1 and gsw were decreased in all species by correcting for gcw.
Discussion
Species-specific leaf conductance characteristics and their implications for A/C i curves
Leaf conductance and stomatal behavior are known to vary across environmental conditions and species (Jones et al. 2009; Cabrerizo and Marañón 2022). Through the characterization of species-specific leaf conductance, researchers can gain valuable insights into the functional traits and adaptations that contribute to the successful adaptation of plants in diverse environments (Mott and Buckley 2000; Franks et al. 2009). For this study, we selected four plant species with distinct leaf conductance characteristics: bell pepper and papaya, exhibiting high glw, citrus with intermediate glw, and magnolia with the lowest glw. gcw values were 1–2 orders of magnitude less than the glw (Table 2). gcw:glw helped make species more comparable in terms of misestimation of Ci, and a ratio of only 0.1 was required to exceed 5% misestimation of Ci whenever Ca was 400 ppm or greater. This ratio could be used to assess the risk of large Ci misestimation, because the risk of misestimation could not be assessed using glw alone. The risk of misestimation of Ci increased with Ca, however, the 5% misestimation point is exceeded at approximately the same glw for Ca of both 400 and 2,000 ppm. This misestimation has implications for the interpretation of A/Ci curves, as demonstrated by the problematic nature of some of the uncorrected SS curves (Fig. 4). Our findings emphasize the necessity of accounting for gcw in accurately estimating Ci and highlight the potential impact of misestimation on the interpretation of A/Ci responses.
Impact of accounting for g cw on A/C i fitted parameters
The cuticle has been known to impact the estimation of photosynthetic parameters by impacting estimates of Ci (Hanson et al. 2016). According to previous reports, gcw influences the calculation of Ci (Boyer 2015a; Tominaga et al. 2018). In the present study, recalculated A/Ci curves (both SS and RACiR) using the gcw values obtained from the red-light method (Márquez et al. 2021) indicate that considering gcw does modify Ci estimates. The extent of the effect varies among species and is more pronounced when the gcw:glw ratio is high or the gsw is low, which results in a decrease in Ci. Lamour et al. (2022) proposed that the ratio of gcw:glw could be important in predicting the misestimation of Ci. The present results support and extend that hypothesis by demonstrating that even high conductance species may have sufficiently high gcw:glw ratios to have strong impacts on the misestimations of Ci. Papaya was the least affected on average, as it exhibited the highest gsw during measurements, whereas magnolia, with low gsw, showed significant changes in most photosynthetic parameters. These findings underscore the importance of gcw in estimating photosynthetic traits, as ignoring gcw can introduce a bias that incorrectly increases estimates of Ci. The data presented in Table 3 and Table 4 indicate a significant increase in Vcmax, Jmax, Rdark, and Citrans1 when the gcw:gsw ratio is high, which could occur in any of the species assessed when gsw was low within the range for that species. Thus, plant conditions of water deficit, supra- or suboptimal temperatures, or other adverse growing conditions are likely to induce sufficiently low gsw as to necessitate the estimation of gcw to estimate accurate photosynthetic parameters.
Constant-ramping A/Ci method improves parameter resolution and avoids some stomatal limitations
In previous studies, comparisons between the RACiR and SS methods have indicated that the A/Ci fitted parameters, Vcmax and Jmax, show no significant differences in a range of species (Stinziano et al. 2017, 2019b; Coursolle et al. 2019; Lawrence et al. 2019; Saathoff and Welles 2021; Lin et al. 2023). In our study, the results of A/Ci curves obtained using both the SS and RACiR methods were similar, with more precise estimates of Citrans1 observed with the RACiR method, as seen in the smaller standard deviation in Citrans1 in RACiR than SS for all species, with and without correction of Ci (Table 3). For example, the reductions in standard deviation represent a 16% decrease in the coefficient of variation (standard deviation/mean) for magnolia (the smallest change) and a 78% decrease in the coefficient of variation for bell pepper (the largest change). However, a notable difference was found in the gsw values, where the SS curves showed a greater decrease in gsw compared to the RACiR curves (Fig. 5). The likely reason for these differences is that the RACiR curves allowed less time for gsw to decrease before reaching the critical threshold. SS curves are measured slowly relative to the time scale of leaf CO2 attenuation of gsw (Merilo et al. 2015). This leads to the reduction of gsw in some curves below that required for reliable estimates of Ci. We do note, however, that the traditional approach to A/Ci curves—starting near atmospheric CO2, going down, then up such as in Busch (2018) may not be the best option for maximizing gsw during the SS. Starting at low CO2 and monotonically increasing CO2 may result in better A/Ci estimates (Sharkey 2019), though our initial attempts were not successful with this approach in terms of avoiding the gsw crash in citrus and magnolia.
To assess the A/Ci relationship, it is ideal to achieve a wide range of Ci values. The RACiR method achieved as wide a range of Ci as the SS method, though low conductance magnolia limited the Ci range achieved (Fig. 2). These findings align with the results reported by Lin et al. (2023), who found that measuring SS A/Ci and RACiR curves in low conductance species posed challenges due to their low gsw values. Consequently, driving Ci to a very high level is difficult or even impossible in these species. In the present study, at higher Ca values gsw decreased (Fig. 5). In this case, the advantage of constant ramping lies in that its short time “beats” the speed of stomatal attenuation, affording the ability to avoid stomatal impacts on the shape of the A/Ci curve, making TPU estimation more feasible in low conductance species.
Stomatal attenuation can particularly affect the estimation of TPU, as it may induce changes in A at high Ci that are limited by gsw, rather than by enzymatic limitations, despite the similarity in shape of the curve to the expected shape with TPU limitations, as is seen in Fig. 4 in papaya and bell pepper. Plants are not typically TPU-limited under ambient conditions (Sage and Sharkey 1987; Sharkey 2019; Ellsworth et al. 2015), and TPU limitation is most easily seen by elevating the rate of photosynthesis through increased light or CO2 or decreased O2 partial pressure (Sharkey et al. 1986) such that A is increased by 10% or 20% relative to ambient conditions (Kirschbaum 2011). Our initial comparison was between RACiR and SS fitted photosynthetic parameters with Ca maxima of 900 and 2000 µmol mol−1, respectively. In this comparison, SS curves yielded a greater number of TPU estimates than RACiR curves. However, when RACiR was performed with a maximum Ca of 2000 µmol mol−1, TPU estimates were achieved in all species (Table 4). Thus, constant-ramping methods can consistently estimate TPU when a sufficiently high maximum Ca are used.
In addition to its increased speed, the utilization of a constant ramping technique, such as RACiR, offers the advantage of improved resolution of parameter estimates. This improvement stems from the reduction in parameter uncertainty that can be achieved by having a larger amount of data available for curve fitting (Saathoff and Welles 2021). It has been observed that the accuracy of parameter estimation is directly influenced by both the number of data points and the accuracy of the gas exchange data used. When working with small and noisy data sets, obtaining robust parameter estimates can be particularly challenging (Sharkey et al. 2007; Wang et al. 2017). In our study, the SS A/Ci curve data consisted of only 16 data points, while the RACiR curves comprised significantly higher numbers (270 and 600 data points at 900 and 2000 µmol mol−1 Ca, respectively). Our findings indicate the larger data set obtained by the RACiR technique reduced parameter uncertainty in comparison to SS.
The uncertainty of instrumental noise in glw estimates has the potential to further compound the challenges of low conductance. Accounting for gcw can mitigate misestimation of Ci, and if gcw is accounted for, the potential error due to instrumental noise only exceeds 5% at a gcw: glw ratio of 0.13. However, constant-ramping methods ameliorate the impact of instrumental noise on the estimation of photosynthetic parameters due to the substantial number of measurements collected. While individual measurements of glw may deviate by approximately ± 0.00415 mol m−2 s−1 from the true value, the cumulative effect of numerous measurements diminishes the impact of the error on the overall results. With an increased number of measurements, the net effect tends toward zero, rendering the influence of instrumental noise inconsequential in the approach that employs the larger dataset. This is reflected in the very small impact of the error of glw on parameter estimates in RACiR relative to SS curves (Fig. 3). Thus, accounting for gcw and utilizing constant-ramping methods significantly enhance the accuracy and reliability of A/Ci parameter estimates.
Conclusions
Low conductance presents a challenge to the estimation of photosynthetic parameters via gas exchange due to the misestimation of Ci. This work demonstrates that not accounting for gcw can lead to a misestimation of Ci, which compounds to the estimation of parameters based on Ci, leading to underestimation of Vcmax and Jmax. Instrumental noise can further compound errors when using a steady-state approach to A/Ci measurement. The ratio of gcw:glw is useful in assessing the risk of misestimating Ci. Although this challenge was expected for low-conductance species, this work showed that high-conductance species, like C. papaya, can also exhibit high risk of overestimating Ci. Thus, practices to improve the estimation of Ci include using constant-ramping methods and measuring and accounting for gcw on at least a species basis. Using these approaches will improve the accuracy of A/Ci-based photosynthetic parameter estimation, especially under conditions that reduce gsw.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Boyer JS (2015a) Impact of cuticle on calculations of the CO2 concentration inside leaves. Planta 242(6):1405–1412. https://doi.org/10.1007/s00425-015-2378-1
Boyer JS (2015b) Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. J Exp Bot 66(9):2625–2633
Boyer JS, Wong SC, Farquhar GD (1997) CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol 114(1):185–191
Busch FA (2018) Photosynthetic gas exchange in land plants at the leaf level. In: Covshoff S (ed) Photosynthesis: methods and protocols. Springer, New York, pp 25–44. https://doi.org/10.1007/978-1-4939-7786-4_2
Cabrerizo MJ, Marañón E (2022) Net effect of environmental fluctuations in multiple global-change drivers across the tree of life. Proc Natl Acad Sci 119(32):e2205495119. https://doi.org/10.1073/pnas.2205495119
Coursolle C, Otis Prud’homme G, Lamothe M, Isabel N, (2019) Measuring rapid A-Ci curves in boreal conifers: Black spruce and balsam fir. Front Plant Sci 10:1276
Duursma RA (2015) Plantecophys-an R package for analysing and modelling leaf gas exchange data. PLoS ONE 10(11):e0143346
Ellsworth DS, Crous KY, Lambers H, Cooke J (2015) Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant Cell Environ 38(6):1142–1156
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149(1):78–90. https://doi.org/10.1007/BF00386231
Franks PJ, Drake PL, Beerling DJ (2009) Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ 32(12):1737–1748. https://doi.org/10.1111/j.1365-3040.2009.002031.x
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell Environ 28(7):834–849. https://doi.org/10.1111/j.1365-3040.2005.01333.x
Hanson DT, Stutz SS, Boyer JS (2016) Why small fluxes matter: the case and approaches for improving measurements of photosynthesis and (photo) respiration. J Exp Bot 67(10):3027–3039
Holmgren P, Jarvis PG, Jarvis MS (1965) Resistances to carbon dioxide and water vapour transfer in leaves of different plant species. Physiol Plant 18(3):557–573
Jones HG, Serraj R, Loveys BR, Xiong L, Wheaton A, Price AH (2009) Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct Plant Biol 36(11):978–989. https://doi.org/10.1071/fp09123
Kirschbaum MU (2011) Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol 155(1):117–124. https://doi.org/10.1104/pp.110.166819
Körner C, Bannister P, Mark A (1986) Altitudinal variation in stomatal conductance, nitrogen content and leaf anatomy in different plant life forms in New Zealand. Oecologia, pp 577–588
Kumarathunge DP, Medlyn BE, Drake JE, Tjoelker MG, Aspinwall MJ, Battaglia M, Cano FJ, Carter KR, Cavaleri MA, Cernusak LA, Chambers JQ, Crous KY, De Kauwe MG, Dillaway DN, Dreyer E, Ellsworth DS, Ghannoum O, Han Q, Hikosaka K, Jensen AM, Kelly JWG, Kruger EL, Mercado LM, Onoda Y, Reich PB, Rogers A, Slot M, Smith NG, Tarvainen L, Tissue DT, Togashi HF, Tribuzy ES, Uddling J, Vårhammar A, Wallin G, Warren JM, Way DA (2019) Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol 222(2):768–784
Lamour J, Davidson KJ, Ely KS, Li Q, Serbin SP, Rogers A (2022) New calculations for photosynthesis measurement systems: What’s the impact for physiologists and modelers? New Phytol 233(2):592–598. https://doi.org/10.1111/nph.17762
Lawrence EH, Stinziano JR, Hanson DT (2019) Using the rapid A-Ci response (RACiR) in the Li-Cor 6400 to measure developmental gradients of photosynthetic capacity in poplar. Plant Cell Environ 42(2):740–750. https://doi.org/10.1111/pce.13436
Lin Q, Tian D, Zhao C, Wang B, Yan Z, Stocker BD, Ye Li, Fang J (2023) Application of the rapid leaf A-Ci response (RACiR) technique: examples from evergreen broadleaved species. Photosynth Res 155(2):139–146. https://doi.org/10.1007/s11120-022-00980-1
Lombardozzi DL, Smith NG, Cheng SJ, Dukes JS, Sharkey TD, Rogers A, Fisher R, Bonan GB (2018) Triose phosphate limitation in photosynthesis models reduces leaf photosynthesis and global terrestrial carbon storage. Environ Res Lett 13(7):074025
Márquez DA, Stuart-Williams H, Farquhar GD (2021) An improved theory for calculating leaf gas exchange more precisely accounting for small fluxes. Nat Plants 7(3):317–326
Márquez DA, Stuart-Williams H, Farquhar GD, Busch FA (2022) Cuticular conductance of adaxial and abaxial leaf surfaces and its relation to minimum leaf surface conductance. New Phytol 233(1):156–168. https://doi.org/10.1111/nph.17588
Merilo E, Jalakas P, Kollist H, Brosché M (2015) The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol Plant 8(4):657–659
Mott KA, Buckley TN (2000) Patchy stomatal conductance: emergent collective behaviour of stomata. Trends Plant Sci 5(6):258–262. https://doi.org/10.1016/S1360-1385(00)01648-4
Oleson KW, Lawrence DM, Bonan GB, Drewniak B, Huang M, Koven CD, Levis S, Li F, Riley WJ, Subin ZM, Swenson SC, Thornton PE (2013) Technical Description of version 4.5 of the Community Land Model (CLM). National Center For Atmospheric Research, Boulder, Colorado, pp 80307–3000
Pilon C, Snider JL, Sobolev V, Chastain DR, Sorensen RB, Meeks CD, Massa AN, Walk T, Singh B, Earl HJ (2018) Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J Plant Physiol 231:124–134
R Core Team R (2013) R: A language and environment for statistical computing. https://www.R-project.org
Radin JW, Lu Z, Percy RG, Zeiger E (1994) Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Proc Natl Acad Sci 91(15):7217–7221
Rogers A, Medlyn BE, Dukes JS, Bonan G, Von Caemmerer S, Dietze MC, Kattge J, Leakey AD, Mercado LM, Niinemets Ü (2017) A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol 213(1):22–42
Saathoff AJ, Welles J (2021) Gas exchange measurements in the unsteady state. Plant Cell Environ 44(11):3509–3523. https://doi.org/10.1111/pce.14178
Sage RF, Sharkey TD (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiol 84(3):658–664
Sharkey TD (2019) Is triose phosphate utilization important for understanding photosynthesis? J Exp Bot 70(20):5521–5525
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30(9):1035–1040
Sharkey TD, Stitt M, Heineke D, Gerhardt R, Raschke K, Heldt HW (1986) Limitation of photosynthesis by carbon metabolism: II. O2-insensitive CO2 uptake results from limitation of triose phosphate utilization. Plant Physiol 81(4):1123–1129
Smith NG, McNellis R, Dukes JS (2020) No acclimation: instantaneous responses to temperature maintain homeostatic photosynthetic rates under experimental warming across a precipitation gradient in Ulmus americana. AoB Plants 12(4):plaa027
Steppe K, Dzikiti S, Lemeur R, Milford JR (2006) Stomatal oscillations in orange trees under natural climatic conditions. Ann Bot 97(5):831–835
Rapid A/Ci response analysis with racir. v2.0.0 (2020) https://cran.r-project.org/web/packages/racir/index.html
Stinziano JR, McDermitt DK, Lynch DJ, Saathoff AJ, Morgan PB, Hanson DT (2019a) The rapid A/Ci response. New Phytol 221(2):625–627
Stinziano JR, McDermitt DK, Lynch DJ, Saathoff AJ, Morgan PB, Hanson DT (2019b) The rapid A/Ci response: a guide to best practices. New Phytol 221(2):625–627. https://doi.org/10.1111/nph.15383
Stinziano JR, Morgan PB, Lynch DJ, Saathoff AJ, McDermitt DK, Hanson DT (2017) The rapid A-Ci response: photosynthesis in the phenomic era. Plant Cell Environ 40(8):1256–1262. https://doi.org/10.1111/pce.12911
Stinziano JR, Tominaga J, Hanson DT (2020) Where in the leaf is intercellular CO2 (Ci)? Considerations and recommendations for assessing gaseous diffusion in leaves. bioRxiv:2020.2005. 2005.079053
Stinziano JR, Way DA (2017) Autumn photosynthetic decline and growth cessation in seedlings of white spruce are decoupled under warming and photoperiod manipulations. Plant, Cell Environ 40(8):1296–1316
Tejera-Nieves M, Seong DY, Reist L, Walker BJ (2024) The Dynamic Assimilation Technique measures photosynthetic CO2 response curves with similar fidelity as steady-state approaches in half the time. J Experim Bot erae057
Tezara W, Fernández M, Donoso C, Herrera A (1998) Seasonal changes in photosynthesis and stomatal conductance of five plant species from a semiarid ecosystem. Photosynthetica 35:399–410
Tominaga J, Shimada H, Kawamitsu Y (2018) Direct measurement of intercellular CO2 concentration in a gas-exchange system resolves overestimation using the standard method. J Exp Bot 69(8):1981–1991. https://doi.org/10.1093/jxb/ery044
Vincent C, Rowland D, Schaffer B (2017) Primed acclimation of papaya increases short-term water use but does not confer long-term drought tolerance. HortScience 52(3):441–449
Sv VC, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Wang Q, Chun JA, Fleisher D, Reddy V, Timlin D, Resop J (2017) Parameter estimation of the Farquhar—Von Caemmerer—Berry biochemical model from photosynthetic carbon dioxide response curves. Sustainability 9(7):1288
Way DA, Sage RF (2008) Thermal acclimation of photosynthesis in black spruce [Picea mariana (Mill.) BSP]. Plant Cell Environ 31(9):1250–1262
Zhu L, Cernusak LA, Song X (2020) Dynamic responses of gas exchange and photochemistry to heat interference during drought in wheat and sorghum. Funct Plant Biol 47(7):611–627
Acknowledgements
We gratefully acknowledge the support and funding provided by the United States Department of Agriculture, National Institute of Food and Agriculture, Agricultural and Food Research Initiative project 2021-67013-33795 for funding this research. We would also like to express our sincere appreciation to Jason Hupp for his invaluable technical support and expertise with the LI-6800. Additionally, we extend our thanks to Florian A. Busch for his valuable insights and feedback on the red-light method and to Berkley Walker for helpful discussions.
Author information
Authors and Affiliations
Contributions
CV and JS planned and designed the research. SBH and MOP conducted the experiments. CV and SBH processed the data. SBH performed the statistical analysis. CV and SBH drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussain, S.B., Stinziano, J., Pierre, M.O. et al. Accurate photosynthetic parameter estimation at low stomatal conductance: effects of cuticular conductance and instrumental noise. Photosynth Res 160, 111–124 (2024). https://doi.org/10.1007/s11120-024-01092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-024-01092-8