Abstract
Different light spectra from light-emitting diodes (LEDs) trigger species-specific adaptive responses in plants. We exposed Artemisia argyi (A. argyi) to four LED spectra: white (the control group), monochromatic red light (R), monochromatic blue light (B), or a mixture of R and B light of photon flux density ratio is 3 (RB), with equivalent photoperiod (14 h) and light intensity (160 μmol s−1 m−2). R light accelerated photomorphogenesis but decreased biomass, while B light significantly increased leaf area and short-term exposure (7 days) to B light increased total phenols and flavonoids. HPLC identified chlorogenic acid, 3,5-dicaffeoylquinic acid, gallic acid, jaceosidin, eupatilin, and taxol compounds, with RB and R light significantly accumulating chlorogenic acid, 3,5-dicaffeoylquinic acid, and gallic acid, and B light promoting jaceosidin, eupatilin, and taxol. OJIP measurements showed that B light had the least effect on the effective quantum yield ΦPSII, with higher rETR(II), Fv/Fm, qL and PIabs, followed by RB light. R light led to faster photomorphology but lower biomass than RB and B lights and produced the most inadaptability, as shown by reduced ΦPSII and enlarged ΦNPQ and ΦNO. Overall, short-term B light promoted secondary metabolite production while maintaining effective quantum yield and less energy dissipation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agriculture production techniques are always being updated and enhanced as human society progresses. Among them, light has a significant impact on the development, productivity, and quality of crops (Dou et al. 2017). It is challenging to meet the needs of modern agriculture using traditional natural light because it is generally constrained by variables like climate, season, and weather. As a new kind of artificial light source, LED (Light Emitting Diode) lights have gradually grown in significance in contemporary agriculture. With the ability to regulate light quality, irradiance, and photoperiod, LEDs offer the advantage of being able to create ideal growth conditions for crops, resulting in high efficiency, low consumption, high yield, and high quality (Guimara et al. 2022). Various types of lights might have distinct effects. Among the different light wavelengths, blue light (425–490 nm) and red light (610–720 nm) are the main spectra absorbed by the chlorophyll in plant cells (Bartucca et al. 2020; Pacheco et al. 2016).
The morphological, physiological, and biochemical characteristics of plants are significantly influenced by the varied wavelengths of LEDs (Demir et al. 2023). Blue light promoted lateral stem growth and increase the total leaf area (Chen et al. 2019), but it also reduced the net photosynthetic rate (Pn) of many species, such as chrysanthemum plantlets (Kim et al. 2004), Withania somnifera (L.) plantlets (Lee et al. 2007). In addition, blue light has been shown to induce physiological activities in cucumber, such as stomatal opening and chloroplast movement through phototropes (Wang et al. 2009). Conversely, red light has been found to promote taller plant heights and smaller leaves in tomato seedlings and lettuce (Lin et al. 2013; Nanya et al. 2012).
Artemisia argyi (A. argyi), a perennial herbaceous plant belonging to the Asteraceae family, is widely distributed in China and has been traditionally used for the treatment of various ailments, including dysmenorrhea, diarrhoea, malaria and inflammatory processes. Its medicinal characteristics are attributed to its active ingredients, including volatile oil, terpenes, flavonoids and phenolic acids (Cui et al. 2022). Moreover, recent studies have shown that moxibustion, a medicinal product made from A. argyi leaves, played a crucial role in the prevention and treatment of Corona Virus Disease 2019 (COVID-19) (Teng et al. 2020) and the prognostic treatment of splenic and pulmonary weakness during the convalescence (Wang et al. 2022). Therefore, research on A. argyi focuses mostly on the analysis of medicinal ingredients and the identification of their functions. However, limited research has been conducted on the effects of long-term continuous LED light exposure on the growth, development, and medicinal properties of A. argyi.
Metabolic profiling has proven to be a powerful tool for gaining insights into functional biology. For instance, blue light induced higher levels of anthocyanin accumulation in red lettuce, strawberry fruit and grape (Li et al. 2017; Samuoliene et al. 2012; Saure 1990); as well as increase the contents of chlorogenic acid, gallic acid, ferulic acid, and other phenolic substances in pea seedlings (Liu et al. 2016). Moreover, red light mainly promotes stem growth by regulating gibberellin biosynthesis in “Manicure Finger” (Vitis vinifera L.) grape plantlets (Li et al. 2017), and exposure to both red and blue light (RB) for three months stimulated carotenoid biosynthesis, which is a sign of a slower decay of product compounds after harvest in tomatoes (Appolloni et al. 2023). Although several studies have focused on the effect of light environments on crop yield and quality, limited research has been conducted on the dynamic responses of growth and metabolism to long-term continuous light exposure (Van Gestel et al. 2005a, b). Therefore, the present study aims to determine the dynamic changes of the total flavonoids (TF) and phenols (TP) in A. argyi. High-performance liquid chromatography (HPLC) will be used to identify and quantify critical compounds, including chlorogenic acid, 3,5-dicaffeoylquinic acid, gallic acid, jaceosidin, eupatilin, and taxol triterpenes.
Chlorophyll fluorescence is a probe of photosynthesis in vivo, which is significantly affected by Photosystem II (PSII) and its electron transfer process (Evans et al. 2017). All oxygenic photosynthetic material investigated so far using this method shows the polyphasic rise with the basic steps from the ‘origin’ (O) through two ‘inflections’ (designated as J, and I) to a ‘peak’ fluorescence level (P) (Strasser et al. 2004). Photochemical reactions consume most of the energy, making them highly sensitive to various stress conditions and thus useful for assessing the photosynthetic capacity of various plants (Petcu et al. 2014; Wang et al. 2010). In chrysanthemum and three ornamental potted plants, blue light resulted in the highest Fv/Fm and ΦPSII (Zheng and Van Labeke 2017). In potato seedings, blue and red light resulted in the Fv/Fm, ΦPSII, qL and rETR(II) increased and decreased, respectively (Chen et al. 2021). However, in Eutrema salsugineum callus cells, Fv/Fm and ΦPSII under red light were significantly higher than those under blue light (Pashkovskiy et al. 2018), suggesting that the effects of light quality on different species cannot be generalized. Therefore, we will use chlorophyll fluorescence parameters as a probe to reveal the photosynthetic mechanisms of A. argyi under different LED light conditions.
Materials and methods
Plant materials
Healthy, fresh shoot stems (approximately 5–10 cm in length) of Artemisia argyi (A. argyi, family Asteraceae) were collected from Henan Province, China. After removing the leaves, stems were washed thoroughly under running water for 1 h. The explants were then transferred into a sterile culture bottle and immersed in 75% (v/v) ethanol solution for 30 s, followed by soaking in 0.1% (w/v) HgCl2 for 3 min on a clean bench (SW-CJ-2D, Suzhou Purification Equipment Co., Ltd). Finally, the stems were washed 5 times with sterilized distilled water to remove the residual sterilant. The disinfected shoot stems, which were cut into small segments (0.5–1.0 cm) with axillary buds, were used as explants for in vitro adventitious root induction.
Basal media and culture conditions
The explants were cultured in adventitious root induction medium based on 1/2 MS basal medium (Murashige and Skoog 1962), fortified with 6.8 g·L−1 agar and 30 g·L−1 sucrose. The pH of the media was adjusted to 5.8 with 1 N NaOH or 1 N HCl and then autoclaved at 121 °C and 105 kPa for 20 min. The cultures were cultivated at 25 ± 2 °C under a 14 h photoperiod with a light intensity of 160 μmol s−1 m−2 (Xiamen Rural Hui Photoelectric Technology Co. Ltd.). All chemicals and reagents mentioned above were analytical grade and purchased from Sigma Chemical Company, USA.
A. argyi seedlings were grown under white fluorescent lamps until the emergence of 3–4 true leaves, then exposed to 21 days of different LED light treatments. These included white LED light (White, as the control group), blue light (B) with a maximum intensity at 455 nm, red light (R) with a maximum intensity at 660 nm, and a combination of red and blue light (R/B light photosynthetic photon flux density ratio is 3, RB). The light intensity and spectral distribution were measured using SpectraPen LM 510-H/UVIS (The Czech Republic) (Fig. 1).
Growth parameters
The plant height, adventitious root number (excluding fibrous roots), and root length were measured after 7, 14, and 21 days of culture. Meanwhile, after 21 days of continuous light exposure, the leaf area was measured using Image J software. The aboveground part of A. argyi was harvested from 4 individuals per replicate and weighed for fresh weight. The samples were then oven-dried at 80 °C until a constant mass was achieved to determine dry weight (g).
Total flavonoids and phenols
After 7, 14 and 21 days of culture, 200 mg leaves were taken as samples and ground into powder in liquid nitrogen to determine the contents of TF and TP, respectively. The TF content was determined using the protocols of Prochazkova et al. (2011) by the NaNO2–Al(NO3)3–NaOH method, which was slightly improved. Rutin was used as the standard. The absorbance at 510 nm was measured by a Scandrop spectrophotometer (Analytikjena, Germany). The yield of TF was calculated.
The TP content was determined using Folin-Ciocalteu’s (Ferreira et al. 2017) reagent according to previous protocols, which was slightly improved. Gallic acid was used as the standard. The absorbance at 765 nm was measured by a Scandrop spectrophotometer (Analytikjena, Germany). The yield of TP was calculated.
HPLC analysis
After 7 days of culture, 200 mg samples were ground into powder in liquid nitrogen. The extraction was performed with 70% (v/w) ethanol overnight. The samples were then sonicated by ultrasonic homogenizer at 300W, for 30 min (Ningbo Science Biotechnology Co., Ltd., SCIENTZ-IID, China). Impurities were removed by centrifugation (BECKMAN COULTER, Life Sciences, USA) at 4 °C, 10,000×g. The supernatant was evaporated to dryness by freeze dryer (Ningbo Science Biotechnology Co., Ltd., SCIENTZ-IID, China) and dissolved in 1 mL chromatographic grade methanol.
The phenolic content (chlorogenic acid, 3,5-dicaffeoylquinic acid, gallic acid), flavonoids (jaceosidin, eupatilin) and diterpene alkaloids (Taxol) were analysed by Ultimate-3000HPLC (Thermo Fisher Scientific, Massachusetts, USA) with a C18 analytical column (Gemini 150 × 2.0 mm, particle size 3 μm; Phenomenex). The analytical conditions were optimized and validated as follows.
The mobile phase was 0.5% acetic acid in water (a) and acetonitrile (b). The gradient program was started with 10%b until 15 min, and changed to obtain 20%, 40%, 50%, 90%, 90%, 10% at 20, 30, 40, 47,55 and 56 min, respectively. The injection volume was 20 μL and the flow rate was 0.8 mL/min. The chromatograms were registered at 241 nm (taxol), 299 nm (gallic acid), 326 nm (chlorogenic acid, 3,5-dicaffeoylquinic acid), and 346 nm (jaceosidin, eupatilin). The peaks were confirmed using commercial pure standards (Aladdin, China). Data integration and analysis were performed using MassHunter Workstation software.
Chlorophyll fluorescence
The Fluorpen FP110 (Czech Republic), was used to measure the chlorophyll fluorescence-related parameters in leaves. The third fully expanded leaves was dark adapted with leaf clips for 20 min. The fluorescence was induced by visible light with a wavelength of 455 nm. Flashpulse, superpulse and actinicpulse were set to 0.03 μmol·m−2·s−1, 1600 μmol·m−2·s−1 and 160 μmol·m−2·s−1, respectively. The fluorescence parameters used in this paper are calculated according to the formula in Table 1. OJIP curves need to be standardized to eliminate the influence of unnecessary factors, such as leaf thickness, pigments, and leaf surface appendages, on curve differences.
where Ft is the amplitude of the OJIP curve corresponding to time t. Vt is the amplitude of the standardized OJIP curve; ΔVt is the difference between the OJIP curve of the standardized treatment group and the White group.
Statistical analysis
Data are reported as the means ± SDs, biochemical indices were determined by three biological repetitions and three technical repetitions, the average value of each test trial was analysed by one-way ANOVA, and Dunnett's T3 multiple comparison or Duncan multiple range test was used for significant difference analysis at P < 0.05 using GraphPad Prism 9 (GraphPad, San Diego, CA, United States). In the figures, *, **, *** and **** indicate significant differences at the 0.05, 0.01, 0.001 and 0.0001 levels of confidence, respectively, while no label indicates no significance.
Results
Growth parameters
The results showed that there were no significant differences in root number between the B, RB and R lights on the 7th and 14th days (8.17 cm and 10.67 cm for B; 8.50 cm and 10.33 cm for RB; 9.50 cm and 9.83 cm for R), but all were significantly higher than the control group (4.08 cm and 6.83 cm, respectively). On the 21st day, the R light treatment had the highest number of roots (24.00 cm), followed by the RB light treatment (18.17 cm), while the B light treatment (12.67 cm) and control (14.17 cm) did not show significant differences (Fig. 2a).
In terms of root length, R light had an immediate effect on promoting root elongation (2.14 cm) on the 7th day. However, on the 14th day, no differences were observed between lighting treatments and the control group. On the 21st day, both R and RB light had a positive effect on root length, with the largest root length observed in these treatments (5.58 cm and 5.55 cm, respectively) compared to the control group (4.93 cm) (Fig. 2b).
In terms of plant height, there were no observable differences between the lighting treatments and the control on the 7th day. On the 14th day, the plant height of R (3.46 cm) and RB light (3.13 cm) was higher than that of B light (2.28 cm) and the control (2.40 cm). On the 21st, the plant height under R light (6.38 cm) was the largest, followed by RB light (5.19 cm) and the control (4.03 cm), while the plant height under B light (2.82 cm) was the smallest (Fig. 2c, e).
In terms of leaf area, all lighting treatments increased the leaf area, B light (3.99 ± 0.43 cm) being the most effective, followed by R (3.40 ± 0.31 cm) and RB light (3.23 ± 0.18 cm) (Fig. 2D). The fresh weight of lighting treatments all were higher than that of the CK (10.25 ± 0.20 g), but the dry matter concentration was the lowest under R light (16.47 ± 1.12%), and there was no significant difference between RB (14.68 ± 0.93%), B light (16.47 ± 1.12%) and the CK (15.13 ± 0.70%) (Fig. 3).
Total flavonoids and phenols
Biochemical analysis showed significant differences among treatments in the case of TF and TP content. In terms of TF, on the 7th day, the B light treatment had the highest content (5.354 ± 0.57 mg g−1 FW), which was much higher than that of the control group (1.57 ± 0.17 mg g−1 FW), followed by the R light treatment (1.38 ± 0.31 mg g−1 FW). Subsequently, the TF under B light (2.70 ± 0.05 mg g−1 FW) continuously decreased with time, eventually reaching the same as the CK (2.36 ± 0.10 mg g−1 FW).
The RB light treatment did not result in any significant difference and consistently had no difference from the CK (see Fig. 4a).
On the 7th day, the TP content was found to be highest in the B light group (0.25 ± 0.02 mg g−1 FW), which was 0.17 mg higher than the control group (0.08 ± 0.01 mg g−1 FW). Subsequently, TP content decreased continuously with the increase in cultivation time. Neither the R nor the RB light conditions showed any stimulatory effect on TP accumulation. The TP accumulation level in all three treatment groups reached its minimum at 21 days (Fig. 4b).
HPLC analysis
The quantitative results of each substance are shown in Table 2. The content of phenols (chlorogenic acid, 3,5-Dicaffeoylquinic acid and gallic acid) significantly increased under RB (186.70 ± 2.30; 252.29 ± 2.10 and 38.36 ± 1.02 μg g−1, respectively) and R (185.93 ± 4.45; 185.52 ± 0.98 and 36.73 ± 0.55 μg g−1, respectively), both of which were higher than that of the CK (106.66 ± 0.10; 86.66 ± 1.31 and 24.30 ± 0.56 μg g−1, respectively), followed by B (151.77 ± 1.81; 114.20 ± 1.57 and 23.61 ± 1.41 μg g−1, respectively).
The content of flavonoids (jaceosidin, eupatilin) increased most significantly under B light (132.34 ± 1.84; 95.82 ± 1.54 μg g−1, respectively), followed by R light (95.27 ± 2.16; 84.26 ± 2.31 μg g−1, respectively), RB light (94.37 ± 0.64; 72.42 ± 2.01 μg g−1, respectively), and the lowest content was in the CK (52.72 ± 1.09; 61.66 ± 0.73 μg g−1, respectively). The content of taxol was significantly increased in the B light (68.82 ± 1.32 μg g−1), followed by RB light (62.11 ± 0.97 μg g−1), and the content in the R light (57.56 ± 1.67 μg g−1) was not significantly different from the CK.
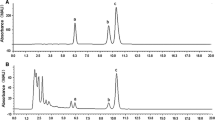
Figure 5 shows HPLC chromatograms of the six tested medicinal ingredients. Taking the maximum absorption wavelength of jaceosidin and eupatilin, i.e., 346 nm as the detection wavelength.
Chlorophyll fluorescence
Figure 6A–C displays the chlorophyll fluorescence kinetic curves of the 7th, 14th and 21st days, respectively. Only under B light, Fo and Fp (Fm) was equal to that of the CK on the 14th day. In all other treatments, Fo and Fp (Fm) was lower than that of the CK (Fig. 7). R light led to a significant increase in the K-phase relative variable fluorescence (Vk) and J-phase relative variable fluorescence (Vj) on the 7th (Fig. 6d, g) and 21st day (Fig. 6f, i). Under B and RB light conditions, chlorophyll fluorescence was consistently lower than that of the CK (Fig. 6a–i).
The OJIP fluorescence kinetics curve (a–c), standardized fluorescence kinetics curve (Vt) (d–f), kinetic difference (ΔVt) (g–i) under four LEDs (white light, B light, RB light, R light), plotted on a logarithmic time scale, of the three monitoring points: A, D, G: culture for 7 days; B, E, H: culture for 14 days; C, F, I: culture for 21 days
Compared to the control group (CK), the ΦPSII of each lighting treatment decreased to varying degrees, with the smallest decrease observed under B light and the largest decrease observed under R light (Fig. 8a). ΦNPQ in each lighting treatment increased significantly, with the most significant increase (0.4564) observed under R light on the 7th day compared to the CK (0.1514). Within 21 days, ΦNO in each lighting treatment gradually increased, with R (0.27 ± 0.02) and RB (0.25 ± 0.02) lights showing the largest increase, both higher than that of the CK (0.21 ± 0.03), followed by B light (0.21 ± 0.02). There was a significant positive correlation between rETR(II) value and ΦPSII. On the 7th day, rETR(II) of each lighting treatment decreased significantly compared to the CK (Fig. 8b).
The NPQ gradually increased over time and peaked on the 21st day. The largest increase was observed under R light (1.197 ± 0.05), followed by RB light (1.168 ± 0.06), while the smallest increase was observed under B light (0.73 ± 0.08), and all were higher than the CK (0.56 ± 0.01). Similarly, the qL value gradually increased, with the largest increase observed under B (0.73 ± 0.03) and RB (0.72 ± 0.00) light, followed by R light (0.68 ± 0.01), all of which were higher than the CK (0.49 ± 0.04). In terms of Fv/Fm, R light was lower than the CK consistently, and no significant difference was observed between RB, B light and the CK on the 7th, 14th and 21st day, respectively (Fig. 9).
Discussion
LEDs, as a dual tool for both research and application of plant photobiology, greatly enrich the content of plant photobiology and expand the methods of regulating light environments. This study investigated the effects of different LED wavelengths and their combinations on the growth, major quality parameters, and chlorophyll fluorescence characteristics of A. argyi.
Photosynthetic pigments strongly absorb R and B lights, making them effective for photosynthesis and thus promoting the growth and development of plants. The results indicate that R light had the most significant effect on accelerating light morphogenesis, as evidenced by increases in root number, root length, and plant height, followed by RB light (Fig. 2a–c). Related research has shown that R light accelerates photomorphogenesis (Kim et al. 2004; Li et al. 2017; Wang et al. 2016). However, the effect of R light on stem elongation seems inconsistent, as some studies suggest that monochromatic R light can inhibit elongation in certain plants (Heo et al. 2002). B light had a significant inhibitory effect on plant height, especially on the 21st day (Fig. 2c, e), while it had the greatest promoting effect on leaf area (Fig. 2d, e). B light inhibits stem elongation and promotes leaf elongation, which has been demonstrated in many species, such as basil plants (O. basilicum) (Hosseini et al. 2019), which is mainly due to the interaction between the B light receptor and photosensitive pigment (Saebo et al. 1995).
Fresh and dry weight are important growth parameters. Previous studies have shown that R light can be detrimental to dry matter accumulation. For example, in broccoli, R light promoted dry matter accumulation more than B and RB light (Demir et al. 2023). In ‘Green Oak Leaf’ lettuce, biomass from R was higher than those from RB, while biomass from B was the lowest (Kwack et al. 2015). However, some studies have found contrasting results, such as Brown et al. (1995), found that biomass under R light was lower than that under B light in pepper. Yorio et al. (2001) showed that dry matter accumulation under R light was lower than that under RB light in lettuce. Similarly, in our study, R light caused a significant decrease in dry matter concentration (Fig. 3), which may be related to excessive growth rates under R light that are not conducive to dry matter accumulation.
In the present work, the content of TF and TP were determined over a period of 21 days. The significant increase in TF and TP mainly occurred within 7 days under B light, but the levels significantly decreased thereafter (Fig. 4). In contrast, there was no noticeable enhancement in TF and TP levels under RB and R lights. Numerous studies have shown that there are significant species differences in the adaptability of plants to continuous R and B light. (Chu et al. 2022; Xu et al. 2014). While some plants are capable of tolerating continuous light to improve their yield and quality, others produce adverse effects, hindering their growth and development (Van Gestel et al. 2005a, b; Zhou et al. 2013). The present results demonstrate that B light is an effective promoter of metabolites in diverse plant species, but for A. argyi, it is limited to short-term irradiation.
The contents of chlorogenic acid, 3,5-dicaffeoylquinic acid, and gallic acid were found to be higher under RB and R light conditions compared to B light (Fig. 5). This is consistent with the findings of Alrifai et al. (2019), who suggested that R light is more effective than B light in promoting chlorogenic acid synthesis through a cryptochrome-mediated pathway. However, in species such as Fragaria vesca (woodland strawberry) and pea (Pisum sativum L.), B light upregulates the synthesis of chlorogenic acid and gallic acid, respectively (Chen et al. 2020; Liu et al. 2016), which presents a clear species specificity. This study is the first to explore the effects of LEDs on the contents of the above three substances. Additionally, B light could significantly increase the contents of jaceosidin, eupatilin, and taxol in the short term, i.e., 7 days.
The shape of the OJIP transient has been found to be sensitive to various stress factors, such as excess light, temperature, and drought, among others (Thach et al. 2007). In the present study, the fluorescence kinetic curve shows a typical polyphase OJIP rise, indicating that the photosynthetic units remained highly active even under limited light wavelengths (Fig. 6a–c). The increase in Vk and Vj are indication of limited electron flux resulting from the accumulation of PSII acceptors (QA and QB, the primary and secondary electron acceptors of PSII, respectively) and the plastoquinone (PQ) pool, leading to a blockade of electron transport (Zampirollo et al. 2021). Notably, R light exposure resulted in a significant increase in the Vk and Vj or ΔVk and ΔVj, confirming the negative effect of R light on photosynthetic units on the 7th and 21st day (Fig. 6d–i).
In this study, A. argyi showed high sensitivity to different LED environments. ΦPSII decreased to various degrees under B, RB and R light within 21 days, among them, B light had the smallest decrease (Fig. 8a). ΦPSII represents the quantum yield of photochemical energy conversion in PSII. The quantum yield of regulated and non-regulated non-photochemical energy loss in PSII represented by ΦNPQ and ΦNO, respectively, which is mainly contributed by PQ in the closed state of PSII, and ΦPSII + ΦNPQ + ΦNO = 1. The ratio of the three directions of excitation energy absorbed by PSII can characterize the light energy conversion efficiency and the ability of self-protection under light stress (Klughammer and Schreiber 2008a, b). Here, the results showed that lighting treatments resulted in a decrease in ΦPSII and an increase in ΦNPQ and ΦNO, indicating that more light energy was dissipated into heat and fluorescence by regulatory and non-regulatory dissipation, respectively (Fig. 8a). Besides, the greater increase in ΦNO than ΦNPQ indicates the damage of photosynthetic apparatus by the stress environment (Klughammer and Schreiber 2008a, b). Thus, we found that R light caused a greater increase in ΦNO than ΦNPQ, indicating that R light affected the function of photosynthetic apparatus, especially on the 21st day, followed by RB light, which also explained the decrease in biomass, TP and TF contents. In contrast, B light is more essential for maintaining the activities of PSII and photosynthetic electron transport capacity, this is similar to the results of Miao et al. (2016) in cucumber leaves.
The photosynthetic electron transport chain provides reductants and energy for the dark reaction of photosynthesis. Therefore, rETR(II) was used as an indicator to partially reflect the photosynthetic potential (Ralph and Gademann 2005). Here, the results showed that under lighting treatments, there was a significant decrease in rETR(II) only on the 7th day, which then returned to the normal level, maintaining a stable photosynthetic potential (Fig. 8B), which also explains the maintenance of ΦPSII values at a appropriate level under continuous illumination.
NPQ represents the proportion dissipated as heat, reflecting the photoprotection capability (Johnson et al. 2007; Nilkens et al. 2010). qL represents the proportion used for photochemical reaction, reflecting the level of photosynthetic activity (Kramer et al. 2004). Fv/Fm and PIABS represent the maximum photosynthetic capacity and survival index of plants, respectively. Photoinhibition is commonly measured as a decrease in the Fv/Fm ratio following sufficient dark adaptation (Demmig et al. 1987). Ouzounis et al. (2015) found that Fv/Fm was lower under R light than in the presence of B light in Phalaenopsis. Similarly, in our study, R light resulted in a significant aboriginal reduction in the Fv/Fm and PIabs, as well as an increase in NPQ, indicating a significant decrease in photosynthetic performance and energy utilization efficiency. Conversely, B and RB light significantly increased Fv/Fm and PIABS values. Therefore, we suggest that B light enhanced the photosynthetic rate and reduced the heat dissipation capacity of PSII more than RB light during 21 days of continuous irradiation (Fig. 9). R light inhibited photosynthesis and increased PSII heat dissipation, which may be caused by the reduced carbon assimilation capacity and electron transport rate (Durand et al. 2019).
Conclusion
The research demonstrated that single R light is more conducive to accelerating the light morphogenesis of A. argyi. Short-term single blue light irradiation, i.e., 7 days, was found to be more conducive to promoting the biosynthesis of TF and TP, but it exhibited substance specificity. For example, RB and R light have a more significant upregulation effect on the contents of three phenolic substances,including chlorogenic acid, 3,5-dicaffeoylquinic, and gallic acid. B light had the most significant effect on jaceosidin, eupatilin and taxol. OJIP measurements revealed that B light had the least effect on the effective quantum yield ΦPSII, as indicated by higher rETR(II), Fv/Fm, qL and PIabs, followed by RB light. R light irradiation produced the most significant inadaptability, as indicated by reduced ΦPSII and significantly enlarged regulated non-photochemical energy dissipation ΦNPQ and non-regulated non-photochemical energy dissipation ΦNO. The sensitivity of chlorophyll fluorescence to light environments expands its application in crops quality detection and evaluation, and provides a theoretical basis for LEDs as a artificial light source to improve agricultural productivity.
Data availability
The raw datasets are available from the first author or corresponding author on reasonable request.
References
Alrifai O, Hao XM, Marcone MF, Tsao R (2019) Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J Agric Food Chem 67:6075–6090
Appolloni E, Pennisi G, Paucek I, Cellini A, Crepaldi A, Spinelli F, Gianquinto G, Gabarrell X, Orsini F (2023) Potential application of pre-harvest LED interlighting to improve tomato quality and storability. Postharvest Biol Technol 195:112113
Bartucca ML, Guiducci M, Falcinelli B, Del Buono D, Benincasa P (2020) Blue: red LED light proportion affects vegetative parameters, pigment content, and oxidative status of einkorn (Triticum monococcum L. ssp. monococcum) wheatgrass. J Agric Food Chem 68:8757–8763
Brown CS, Schuerger AC, Sager JC (1995) Growth and photomorphogenesis of pepper plants under red light-emitting-diodes with supplemental blue or far-red lighting. J Am Soc Hortic Sci 120:808–813
Chen L, Yang Y, Jiang Y, Zhao J, Zang H, Wang X, Hu Y, Xue X (2019) RNA-seq analysis reveals differential responses of potato (Solanum tuberosum L.) plantlets cultured in vitro to red, blue, green, and white light-emitting diodes (LEDs). J Plant Growth Regul 38:1412–1427
Chen X, Cai W, Xia J, Yu H, Wang Q, Pang F, Zhao M (2020) Metabolomic and transcriptomic analyses reveal that blue light promotes chlorogenic acid synthesis in strawberry. J Agric Food Chem 68:12485–12492
Chen L-L, Wang H-Y, Gong X-C, Zeng Z-h, Xue X-Z, Hu Y-G (2021) Transcriptome analysis reveals effects of red and blue light-emitting diodes (LEDs) on the growth, chlorophyll fluorescence and endogenous plant hormones of potato (Solanum tuberosum L.) plantlets cultured in vitro. J Integr Agric 20:2914–2931
Chu X, Li R, Wei HY, Liu HL, Mu YH, Jiang HZ, Ma ZY (2022) Determination of total flavonoid and polysaccharide content in Anoectochilus formosanus in response to different light qualities using hyperspectral imaging. Infrared Phys Technol 122:104088
Cui ZH, Li MZ, Han XJ, Liu HY, Li C, Peng HS, Liu DH, Huang XZ, Zhang ZY (2022) Morphogenesis, ultrastructure, and chemical profiling of trichomes in Artemisia argyi H. Lev. & Vaniot (Asteraceae). Planta 255:102
Demir K, Sarikamis G, Seyrek GC (2023) Effect of LED lights on the growth, nutritional quality and glucosinolate content of broccoli, cabbage and radish microgreens. Food Chem 401:134088
Demmig B, Cleland RE, Bjorkman O (1987) Photoinhibition, 77-K chlorophyll fluorescence quenching and phosphorylation of the light-harvesting chlorophyll-protein complex of photosystem-II in soybean leaves. Planta 172:378–385
Dou H, Niu G, Gu M, Masabni JG (2017) Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 3:36
Durand M, Brendel O, Buré C, Le Thiec D (2019) Altered stomatal dynamics induced by changes in irradiance and vapour-pressure deficit under drought: impacts on the whole-plant transpiration efficiency of poplar genotypes. New Phytol 222:1789–1802
Evans JR, Morgan PB, von Caemmerer S (2017) Light quality affects chloroplast electron transport rates estimated from Chl fluorescence measurements. Plant Cell Physiol 58:1652–1660
Ferreira ICFR, Martins N, Barros L (2017) Phenolic compounds and its bioavailability. in vitro bioactive compounds or health promoters? Adv Food Nutr Res 82:1–44
Heo J, Lee C, Chakrabarty D, Paek K (2002) Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a Light-Emitting Diode (LED). Plant Growth Regul 38:225–230
Hosseini A, Mehrjerdi MZ, Aliniaeifard S, Seif M (2019) Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol Mol Biol Plants 25:741–752
Guimara MMR, Mello SdC, de Freitas IS, Silveira FF, Alves MC, Azevedo RA (2022) Supplemental light with different blue and red ratios in the physiology, yield and quality of Impatiens. Sci Hortic 306
Johnson MP, Havaux M, Triantaphylides C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J Biol Chem 282:22605
Kim SJ, Hahn EJ, Heo JW, Paek KY (2004) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
Klughammer C, Schreiber U (2008a) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes 1:27–35
Klughammer C, Schreiber U (2008b) Saturation Pulse method for assessment of energy conversion in PS I. pam application notes
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218
Kwack Y, Kim KK, Hwang H, Chun C (2015) Growth and quality of sprouts of six vegetables cultivated under different light intensity and quality. Hortic Environ Biotechnol 56:437–443
Lee S-H, Tewari RK, Hahn E-J, Paek K-Y (2007) Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. plantlets. Plant Cell Tissue Organ Cult 90:141–151
Li C-X, Xu Z-G, Dong R-Q, Chang S-X, Wang L-Z, Khalil-Ur-Rehman M, Tao J-M (2017) An RNA-Seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red LED light, and white fluorescent light. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00078
Lin K-H, Huang M-Y, Huang W-D, Hsu M-H, Yang Z-W, Yang C-M (2013) The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci Hortic 150:86–91
Liu H, Chen Y, Hu T, Zhang S, Zhang Y, Zhao T, Yu H, Kang Y (2016) The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J Funct Foods 25:459–465
Miao YX, Wang XZ, Gao LH, Chen QY, Qu M (2016) Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J Integr Agric 15:87–100
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nanya K, Ishigami Y, Hikosaka S, Goto E (2012) Effects of blue and red light on stem elongation and flowering of tomato seedlings. In: 7th International symposium on light in horticultural systems. Wageningen, Netherlands, pp 261–266
Nilkens M, Kress E, Lambrev P, Miloslavina Y, Mueller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. BBA-Bioenergetics 1797:466–475
Ouzounis T, Fretté X, Ottosen C-O, Rosenqvist E (2015) Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’ and ‘Purple Star.’ Physiol Plant 154:314–327
Pacheco FV, Avelar RD, Alvarenga ICA, Bertolucci SKV, de Alvarenga AA, Pinto J (2016) Essential oil of monkey-pepper (Piper aduncum L.) cultivated under different light environments. Ind Crops Prod 85:251–257
Pashkovskiy PP, Soshinkova TN, Korolkova DV, Kartashov AV, Zlobin IE, Lyubimov VY, Kreslavski VD, Kuznetsov VV (2018) The effect of light quality on the pro-/antioxidant balance, activity of photosystem II, and expression of light-dependent genes in Eutrema salsugineum callus cells. Photosynth Res 136:199–214
Petcu E, Schitea M, Dragan L (2014) The effect of water stress on stomatal resistance and chlorophyll fluorescence and their association with alfalfa yield. Rom Agric Res 31:113–119
Prochazkova D, Bousova I, Wilhelmova N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82:513–523
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Saebo A, Krekling T, Appelgren M (1995) Light quality affects photosynthesis and leaf anatomy of birch plantlets in-vitro. Plant Cell Tissue Organ Cult 41:177–185
Samuoliene G, Sirtautas R, Brazaityte A, Duchovskis P (2012) LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem 134:1494–1499
Saure MC (1990) External control of anthocyanin formation in apple. Sci Hortic 42:181–218
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer Netherlands, Dordrecht, pp 321–362
Teng Y, Xiong J, Guo Y, Zeng F (2020) The advantage and value of moxibustion in the prevention and treatment of COVID-19. Mod Tradit Chin Med Materia Medica 22:697–700
Thach LB, Shapcott A, Schmidt S, Critchley C (2007) The OJIP fast fluorescence rise characterizes Graptophyllum species and their stress responses. Photosynth Res 94:423–436
Van Gestel NC, Nesbit AD, Gordon EP, Green C, Paré PW, Thompson L, Peffley EB, Tissue DT (2005a) Continuous light may induce photosynthetic downregulation in onion: consequences for growth and biomass partitioning. Physiol Plant 125:235–246
Van Gestel NC, Nesbit AD, Gordon EP, Green C, Pare PW, Thompson L, Peffley EB, Tissue DT (2005b) Continuous light may induce photosynthetic downregulation in onion - consequences for growth and biomass partitioning. Physiol Plant 125:235–246
Wang H, Gu M, Cui J, Shi K, Zhou Y, Yu J (2009) Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J Photochem Photobiol B-Biol 96:30–37
Wang J, Lu W, Tong Y, Yang Q (2016) Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomata! Development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front Plant Sci 7
Wang XF, Liu GH, Yang Q, Hua W, Liu J, Wang HZ (2010) Genetic analysis on oil content in rapeseed (Brassica napus L.). Euphytica 173:17–24
Wang X, Hou Y, Ding L, Zheng X, Sheng Y, Yu Q, Bi X, Yang J (2022) Moxibustion for anorexia in COVID-19: a protocol for systematic review and meta-analysis. Medicine 101:e28894
Xu F, Cao S, Shi L, Chen W, Su X, Yang Z (2014) Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J Agric Food Chem 62:4778–4783
Yorio NC, Goins GD, Kagie HR, Wheeler RM, Sager JC (2001) Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 36:380–383
Zampirollo JB, Pinheiro CL, Santos VFd, Braga PCS, Martins JPR, Silva DM, Falqueto AR (2021) Analyses of OJIP transients in leaves of two epiphytic orchids under drought stress. Ornam Hortic 27:556–565
Zheng L, Van Labeke MC (2017) Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front Plant Sci 8:55. https://doi.org/10.3389/fpls.2017.00917
Zhou WL, Liu WK, Yang QC (2013) Reducing nitrate content in lettuce by pre-harvest continuous light delivered by red and blue light-emitting diodes. J Plant Nutr 36:481–490
Funding
The study was financially supported by the National Natural Science Foundation of China (32201521), the Youth Innovation Promotion Association CAS (2021447), the Key Research and Development Program of Anhui Province (202204c06020045), the Chinese Academy of Sciences-Henan Province Achievement transfer and Transformation Project (2022110, 2022211 and 2021P02), the Grant of the President Foundation of Hefei Institutes of Physical Science of Chinese Academy of Sciences (YZJJZX202013), and the Strategic Biological Resources Planning Project of the Chinese Academy of Sciences (KFJ-BRP-007-014).
Author information
Authors and Affiliations
Contributions
PS: Investigation, Methodology, Writing-original draft. SD: Investigation, Formal analysis. DW: Validation. WK and MY: Software. XC and CT: Writing—review. JH: Founding acquisition, Conceptualization, Formal analysis, Writing-review and editing. LW: Conceptualization, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no financial or non-financial interests to declare that are relevant to the content of this article.
Consent for publication
All authors give their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, P., Ding, S., Wang, D. et al. Plant morphology, secondary metabolites and chlorophyll fluorescence of Artemisia argyi under different LED environments. Photosynth Res 159, 153–164 (2024). https://doi.org/10.1007/s11120-023-01026-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-023-01026-w