Abstract
Photosystem II (PSII) contains Ca2+, which is essential to the oxygen-evolving activity of the catalytic Mn4CaO5 complex. Replacement of Ca2+ with other redox-inactive metals results in a loss/decrease of oxygen-evolving activity. To investigate the role of Ca2+ in this catalytic reaction, we investigate artificial Mn3[M]O2 clusters redox-inactive metals [M] ([M] = Mg2+, Ca2+, Zn2+, Sr2+, and Y3+), which were synthesized by Tsui et al. (Nat Chem 5:293, 2013). The experimentally measured redox potentials (Em) of these clusters are best described by the energy of their highest occupied molecular orbitals. Quantum chemical calculations showed that the valence of metals predominantly affects Em(MnIII/IV), whereas the ionic radius of metals affects Em(MnIII/IV) only slightly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
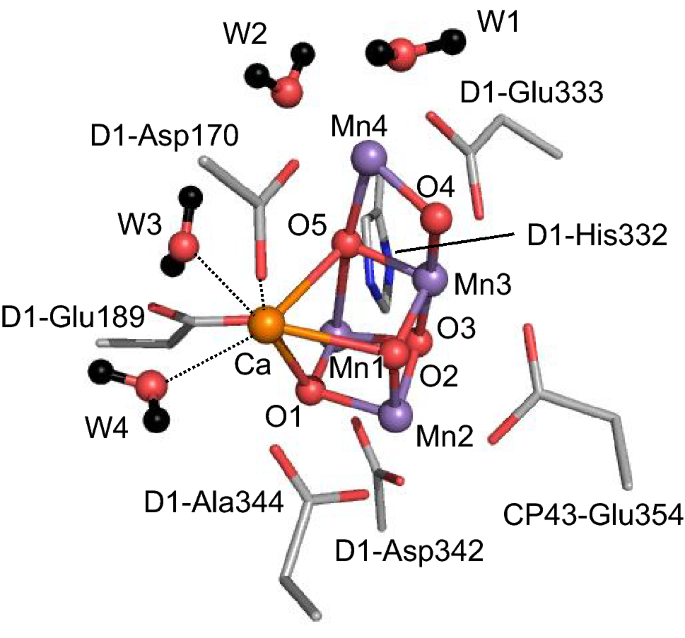
Plants, algae, and cyanobacteria use the water-splitting enzyme photosystem II (PSII) for oxygen evolution. The oxygen evolution proceeds at the oxygen-evolving center, the Mn4CaO5 cluster. The cluster consists of a distorted cubane [Mn1, Mn2, Mn3, four oxygen atoms, and Ca2+] and “dangling” Mn4 (Fig. 1) (Umena et al. 2011). The Mn4CaO5 cluster has two ligand water molecules, W1 and W2, at the Mn4 site and another two ligand water molecules, W3 and W4, at the Ca2+ site (Fig. 1). The catalytic cycle moves through a series of oxidation states, denoted as Sn (n = 0, 1, 2, and 3). As electron transfer occurs, Sn increases. During the catalytic cycle, four electrons from two of the substrate water molecules are removed, and O2 evolves in the S3 to S0 transition (Shen 2015; Cardona and Rutherford 2019).
In the Mn4CaO5 cluster, a redox-inactive Ca2+ is essential for the oxygen evolution activity, as oxygen is not evolved when Ca2+ is removed (Ono and Inoue 1988) or replaced with Dy3+, Cu2+, Cd2+ (Lee et al. 2007), K+, Rb+, and Cs+ (Ono et al. 2001). The Mn4SrO5 cluster can evolve oxygen but the activity is lower than that of the native Mn4CaO5 cluster (Yachandra and Yano 2011). Koua et al. identified that the distance between Sr2+ and W3 (2.6 Å) was longer than that between Ca2+ and W3 (2.4 Å) (Koua et al. 2013) and proposed that the long Sr2+⋯W3 distance contributed to the decrease in the activity upon replacement of Ca2+ with Sr2+.
It was speculated that Ca2+ might be responsible for the distorted cubane structure of the Mn4CaO5 cluster (Kawakami et al. 2011). However, the removal of Ca2+ does not alter the Mn3CaO4 cubane structure (Saito and Ishikita 2014; Siegbahn 2014, 2017), as suggested by the extended X-ray absorption fine structure (EXAFS) and the electron paramagnetic resonance (EPR) measurements (Latimer et al. 1998; Yachandra and Yano 2011; Lohmiller et al. 2012). Note that the Jahn–Teller distortion for Mn(III) ions can be affected by Ca2+ (Yamaguchi et al. 2013). When Ca2+ is removed, the rearrangement of water molecules in the hydrogen-bond (H-bond) network of the redox-active tyrosine (TyrZ) is observed (Saito and Ishikita 2014; Saito et al. 2020a). TyrZ is involved in electron transfer from the Mn4CaO5 cluster to the reaction center chlorophyll PD1. The rearrangement of the H-bond network increases its redox potential (Em(TyrZ)) by ~ 300 mV and inhibits the formation of the downhill electron transfer pathway from the Mn4CaO5 cluster via TyrZ to PD1 (Saito et al. 2020a). Thus, Ca2+ is essential in both maintaining the H-bond network and optimizing electron transfer. The role of Ca2+ as the water binding site can be substituted with H3O+: recent theoretical studies showed that the H-bond network, including the low-barrier H-bond between TyrZ and D1-His190, remains unaltered upon the replacement of Ca2+ with H3O+ (Saito et al. 2020a).
Ca2+ is a prerequisite for the low-barrier H-bond between W1 and D1-Asp61: they form a low-barrier H-bond in native PSII (Kawashima et al. 2018b; Saito et al. 2020a), whereas they cannot form in the absence of Ca2+ (Saito et al. 2020a). That is, Ca2+ decreases pKa(W1) electrostatically to a level of pKa(D1-Asp61) in native PSII, thus forming the low-barrier H-bond and facilitating proton transfer from W1 to D1-Asp61.
So far, the role of the Ca2+ can be summarized as follows: (i) maintaining the TyrZ H-bond network (Saito et al. 2011, 2020a; Kawashima et al. 2018a), including the low-barrier H-bond between TyrZ and D1-His190 (Kawashima et al. 2018b; Saito et al. 2020a); (ii) optimizing Em(TyrZ) in the electron transfer cascade (Saito et al. 2020a); and (iii) electrostatically decreasing pKa(W1) and facilitating proton transfer via the low-barrier H-bond with D1-Asp61 (Saito et al. 2020a).
Althogh it was proposed that Ca2+ might electrostatically affect the properties of the cluster (e.g., pKa and Em) (McEvoy and Brudvig 2006), the replacements of Ca2+ with H2O and H3O+ lead to different Em(MnIII/IV) values due to different H-bond patterns in PSII (Saito et al. 2020a). Artificial Mn clusters with redox-inactive metals (Zhang et al. 2015; Mukherjee et al. 2012; Tsui et al. 2013; Kanady et al. 2013; Tsui and Agapie 2013; Lin et al. 2015) may serve as reference model systems since the corresponding H-bond network is absent. Tsui et al. synthesized artificial Mn3[M]O2 and clusters with redox-inactive metals [M] ([M] = Mg+, Ca2+, Zn2+, Sr2+, and Y3+) (Fig. 2), showing that Em(MnIII/IV) depends on the Lewis acidity of [M] (i.e., the pKa of aqua complexes of [M]) (Tsui et al. 2013). A similar correlation between the ligand-to-metal charge transfer energy (related to Em) and the Lewis acidity has also been reported in the Fe and Mn complexes (Bang et al. 2014; Krewald et al. 2016).
Structure of the Mn3[M]O2 cluster for [M] = Ca2+, Sr2+, and Y3+ (Tsui et al. 2013) a Chemical structure. b Three-dimensional structure. For the detail of ligands, see Table S1
Em can be calculated as the free energy difference between the oxidized and reduced states, including the entropic effect of the solvent (Marenich et al. 2014; Pitari et al. 2015; Amin et al. 2013; Krewald et al. 2016). Em also correlates with the ionization potential as shown for various complexes, including Mn complexes (Marenich et al. 2014; Krewald et al. 2016). The ionization potential can be regarded as the free energy difference between the oxidized and the reduced states when the reorganization effect upon the redox reaction (including the electronic relaxation, the solvent reorganization, and the structural change of the molecule) is neglective. As the ionization potential (or the electronic affinity) is correlated with the energy levels of the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) in density functional theory (DFT) (Kohn–Sham orbital) (Zhang and Musgrave 2007), Em should be calculated based on the HOMO or LUMO energy calculated using DFT. An electron releases from the HOMO upon oxidation, whereas an electron enters the LUMO upon reduction. Thus, the HOMO energy corresponds to the potential for one-electron oxidation, and the LUMO energy corresponds to the potential for one-electron reduction. When the redox reaction is reversible, the midpoint potential Em is located at the midpoint between the oxidation and reduction potentials, i.e., Em and the two potentials have the same tendency. Indeed, the Em of quinones can be determined based on the LUMO energy (Ishikita and Saito 2020) as accurately as the free energy difference (Kishi et al. 2017). For complexes that include transition metals, high correlations between the HOMO and/or LUMO energy and the experimentally measured Em value were observed [e.g., organic compounds (Mendez-Hernandez et al. 2013), β-diketones complexes, including Mn and Fe (Conradie 2015), and FeCo proteins (Dance 2006)]. For the natural Mn4CaO5 cluster in the PSII protein environment, Em can be determined based on the HOMO energy (Mandal et al. 2020; Saito et al. 2020a, b). Here we calculate the Em values of the artificial clusters (Tsui et al. 2013; Tsui and Agapie 2013) based on the HOMO energy and explain how the redox-inactive metal affects Em(MnIII/IV).
Computational details
The crystal structures of synthetic Mn3[M]O2 clusters ([M] = Na+, Sr2+, Ca2+, Zn2+, and Y3+) (Tsui et al. 2013) and Mn3[M]O4 clusters ([M] = Sr2+, Ca2+, Zn2+, Mn3+, Sc3+ and Y3+) (Tsui and Agapie 2013) were used as the basis for geometry optimization using unrestricted DFT (UDFT), with the B3LYP functional and LACVP* basis set (for optimized structures, see Supporting Information). For efficiency, the cluster was considered to comprise ferromagnetically coupled Mn atoms, (Tsui et al. 2013) where the total spin, S, was 12/2 for the Mn3[M]O2 cluster and 10/2 for the Mn3[M]O4 cluster. We note that, in the calculation of the native Mn4CaO5 of PSII, the difference in S (e.g., S = 1/2 in S2 (Zimmermann and Rutherford 1986), high, low, ferromagnetic, and antiferromagnetic) did not affect the (i) resulting geometry (Ames et al. 2011; Isobe et al. 2012), (ii) potential energy profile of proton transfer (Kawashima et al. 2018b), (iii) redox potential of each Mn site (Mandal et al. 2020), or (iv) pKa values of ligand water molecules W1–W4 (Saito et al. 2020c). The resulting oxidation states for three Mn atoms were Mn(III)3 and Mn(IV)Mn(III)2 for the Mn3[M]O2 and the Mn3[M]O4 clusters, respectively (for atomic spin density, see Table S2). Em(Mn1III/IV) was calculated from the HOMO energies, since the value of Em for one-electron oxidation is correlated with the energy of the highest occupied molecular orbital (HOMO) (Mendez-Hernandez et al. 2013; Igarashi and Seefeldt 2003; Mandal et al. 2020). Using the optimized geometries in vacuum, the HOMO energy (EHOMO) was calculated in dichloromethane (CH2Cl2, dielectric constant 8.93) using the polarizable continuum model (PCM). All calculations were performed with Jaguar program [Schrödinger, LLC, 2012, New York]. The initial-guess wavefunctions were obtained using the ligand field theory (Vacek et al. 1999) implemented in the Jaguar program.
Results and discussion
The calculated EHOMO values for the artificial Mn3[M]O2 clusters ([M] = Na+, Sr2+, Ca2+, Zn2+, and Y3+) show a correlation with the experimentally measured Em(MnIII/IV) values (Fig. 3) in CH2Cl2 and are best fitted to Eq. (1).
where Fc/Fc+ denotes ferrocene electrode. A similar correlation is also observed in the Mn3[M]O4 cubane clusters ([M] = Sr2+, Ca2+, Zn2+, Mn3+, Sc3+, and Y3+) (Tsui and Agapie 2013) (Fig. S1). The coefficient of −0.302 in Eq. (1) is the conversion factor from MO energy to Em, which may be associated with the solvation effect (Schmidt am Busch and Knapp 2005), whereas the offset of −1.710 V is associated with a difference between the absolute electrode potential and the Fc/Fc+ electrode potential and liquid junction potential (Kishi et al. 2017). These factors depend on the size and the net charge of the QM system, the solvent, and the reference electrode (e.g., see the caption of Fig. S1). Thus, Eq. (1) is applicable only to similar molecular groups (e.g., artificial Mn3[M]O2 clusters).
Experimentally measured Em(MnIII/IV) values in CH2Cl2 (Tsui and Agapie 2013) and calculated HOMO energy levels (EHOMO) of the Mn3[M]O2 clusters. The coefficient of determination (R2) is 0.96
Using Eq. (1), EHOMO can be converted to Em. The calculated Em values correlate with the experimentally measured Em values (Fig. 4, blue diamonds). In the Mn3[M]O2 clusters synthesized by Tsui, each [M] has different ligand groups (Table S1). Accordingly, the absolute Em values are affected by [M] and the ligand groups. To evaluate the direct influence of electrostatic and the van der Waals interactions with [M] on the Em of the Mn3[M]O2 cluster, the metal [M] was removed from the geometry-optimized Mn3[M]O2 cluster. The calculated Em values for the metal-removed clusters do not correlate with the experimentally measured Em values (Fig. 4, red circles)..
Calculated Em(MnIII/IV) values of the Mn3[M]O2 clusters (blue diamonds) and calculated Em(MnIII/IV) values of the metal-removed Mn3O2 clusters (red circles) plotted with experimentally measured Em(MnIII/IV) values in CH2Cl2 (Tsui and Agapie 2013)
The removal of Y3+ resulted in an increase of 1.5 V in Em, whereas the removal of Na+ resulted in an increase of 0.5 V in Em (Fig. 5a). These results suggest that the valence of [M] is the main factor determining Em. In addition, the removal of a metal with a large radius (e.g., Sr2+) resulted in a large increase in Em, whereas the removal of a metal with a small radius (e.g., Zn2+) resulted in a small increase in Em (Fig. 5b). For metals with the same valence (e.g., Sr2+, Ca2+, and Zn2+), the difference in Em can be explained by the difference in the ionic radius of the redox-inactive metal [M]. As the radius of [M] increases, the distance between [M]2+ and Mn increases, leading to weak electrostatic interactions between Mn and [M]2+. Thus, of the [M]2+ metals, [M] with large radii have a smaller influence on Em. The effect that the ionic radius has on the difference in Em can be explained in terms of the Lewis acidity of [M] because the Lewis acidity decreases with an increase in the ionic radius (Lin et al. 2015).
Shift in Em upon the removal of [M] ([M] = Na+ (red), Sr2+, Ca2+, Zn2+ (green), and Y3+ (blue)) a with respect to the valence (R2 = 0.93) and b with respect to the ionic radius (Shannon 1976) of [M] ([M] = Sr2+, Ca2+, and Zn2+) (R2 = 0.99)
In PSII, Em(Mn1III/IV) of the Mn4CaO5 cluster changes by only ~40 mV even upon the replacement ion of Ca2+ with H3O+ irrespective of the loss of a +1 charge (Saito et al. 2020a). In contrast, Em(MnIII/IV) of the Mn3[M]O2 cluster changes by 450 mV upon the loss of a +1 charge (Fig. 5a). These indicate that the protein environment including the H-bond network (e.g., D1-Asp61, TyrZ, D1-His190, and water molecules) plays a key role in determining the Em(Mn4CaO5) in PSII.
In summary, the quantum-chemically calculated HOMO energies of artificial Mn3[M]O2 clusters ([M] = Na+, Sr2+, Ca2+, Zn2+, and Y3+) correlate with the experimentally measured Em(MnIII/IV) values (Fig. 3). The Em calculation for the metal-deleted Mn3O2 clusters shows that the valence of [M] predominantly affects Em (Fig. 5a), whereas the ionic radius of [M] affects Em only slightly (Fig. 5b).
References
Ames W, Pantazis DA, Krewald V, Cox N, Messinger J, Lubitz W, Neese F (2011) Theoretical evaluation of structural models of the S2 state in the oxygen evolving complex of photosystem II: protonation states and magnetic interactions. J Am Chem Soc 133(49):19743–19757. https://doi.org/10.1021/ja2041805
Amin M, Vogt L, Vassiliev S, Rivalta I, Sultan MM, Bruce D, Brudvig GW, Batista VS, Gunner MR (2013) Electrostatic effects on proton coupled electron transfer in oxomanganese complexes inspired by the oxygen-evolving complex of photosystem II. J Phys Chem B 117(20):6217–6226. https://doi.org/10.1021/jp403321b
Bang S, Lee YM, Hong S, Cho KB, Nishida Y, Seo MS, Sarangi R, Fukuzumi S, Nam W (2014) Redox-inactive metal ions modulate the reactivity and oxygen release of mononuclear non-haem iron(III)-peroxo complexes. Nat Chem 6(10):934–940. https://doi.org/10.1038/nchem.2055
Cardona T, Rutherford AW (2019) Evolution of photochemical reaction centres: more twists? Trends Plant Sci 24(11):1008–1021. https://doi.org/10.1016/j.tplants.2019.06.016
Conradie J (2015) A Frontier orbital energy approach to redox potentials. J Phys Conf Ser 633:012045. https://doi.org/10.1088/1742-6596/633/1/012045
Dance I (2006) The correlation of redox potential, HOMO energy, and oxidation state in metal sulfide clusters and its application to determine the redox level of the FeMo-co active-site cluster of nitrogenase. Inorg Chem 45(13):5084–5091
Igarashi RY, Seefeldt LC (2003) Nitrogen fixation: the mechanism of the Mo-dependent nitrogenase. Crit Rev Biochem Mol Biol 38(4):351–384
Ishikita H, Saito K (2020) Redox potentials of quinones in aqueous solution: relevance to redox potentials in protein environments. In: Wang Q (ed) Microbial photosynthesis. Springer, Singapore, pp 115–120
Isobe H, Shoji M, Yamanaka S, Umena Y, Kawakami K, Kamiya N, Shen JR, Yamaguchi K (2012) Theoretical illumination of water-inserted structures of the CaMn4O5 cluster in the S2 and S3 states of oxygen-evolving complex of photosystem II: full geometry optimizations by B3LYP hybrid density functional. Dalton Trans 41(44):13727–13740
Kanady JS, Mendoza-Cortes JL, Tsui EY, Nielsen RJ, Goddard WA, Agapie T (2013) Oxygen atom transfer and oxidative water incorporation in cuboidal Mn3MOn, complexes based on synthetic, isotopic labeling, and computational studies. J Am Chem Soc 135(3):1073–1082
Kawakami K, Umena Y, Kamiya N, Shen J-R (2011) Structure of the catalytic, inorganic core of oxygen-evolving photosystem II at 1.9 Å resolution. J Photochem Photobiol B 104(1–2):9–18
Kawashima K, Saito K, Ishikita H (2018a) Mechanism of radical formation in the H-bond network of D1-Asn298 in photosystem II. Biochemistry 57(33):4997–5004. https://doi.org/10.1021/acs.biochem.8b00574
Kawashima K, Takaoka T, Kimura H, Saito K, Ishikita H (2018b) O2 evolution and recovery of the water-oxidizing enzyme. Nat Commun 9:1247–1257
Kishi S, Saito K, Kato Y, Ishikita H (2017) Redox potentials of ubiquinone, menaquinone, phylloquinone, and plastoquinone in aqueous solution. Photosynth Res 134(2):193–200. https://doi.org/10.1007/s11120-017-0433-4
Koua FHM, Umena Y, Kawakami K, Shen JR (2013) Structure of Sr-substituted photosystem II at 2.1 Å resolution and its implications in the mechanism of water oxidation. Proc Natl Acad Sci USA 110(10):3889–3894
Krewald V, Neese F, Pantazis DA (2016) Redox potential tuning by redox-inactive cations in nature’s water oxidizing catalyst and synthetic analogues. Phys Chem Chem Phys 18(16):10739–10750. https://doi.org/10.1039/c5cp07213a
Latimer MJ, DeRose VJ, Yachandra VK, Sauer K, Klein MP (1998) Structural effects of calcium depletion on the manganese cluster of photosystem II: determination by X-ray absorption spectroscopy. J Phys Chem B 102:8257–8265
Lee CI, Lakshmi KV, Brudvig GW (2007) Probing the functional role of Ca2+ in the oxygen-evolving complex of photosystem II by metal ion inhibition. Biochemistry 46(11):3211–3223. https://doi.org/10.1021/bi062033i
Lin PH, Takase MK, Agapie T (2015) Investigations of the effect of the non-manganese metal in heterometallic-oxido cluster models of the oxygen evolving complex of photosystem II: lanthanides as substitutes for calcium. Inorg Chem 54(1):59–64
Lohmiller T, Cox N, Su JH, Messinger J, Lubitz W (2012) The basic properties of the electronic structure of the oxygen-evolving complex of photosystem II are not perturbed by Ca2+ removal. J Biol Chem 287(29):24721–24733
Mandal M, Kawashima K, Saito K, Ishikita H (2020) Redox potential of the oxygen-evolving complex in the electron transfer cascade of photosystem II. J Chem Phys Lett 11(1):249–255
Marenich AV, Ho J, Coote ML, Cramer CJ, Truhlar DG (2014) Computational electrochemistry: prediction of liquid-phase reduction potentials. Phys Chem Chem Phys 16(29):15068–15106. https://doi.org/10.1039/c4cp01572j
McEvoy JP, Brudvig GW (2006) Water-splitting chemistry of photosystem II. Chem Rev 106(11):4455–4483
Mendez-Hernandez DD, Tarakeshwar P, Gust D, Moore TA, Moore AL, Mujica V (2013) Simple and accurate correlation of experimental redox potentials and DFT-calculated HOMO/LUMO energies of polycyclic aromatic hydrocarbons. J Mol Model 19(7):2845–2848
Mukherjee S, Stull JA, Yano J, Stamatatos TC, Pringouri K, Stich TA, Abboud KA, Britt RD, Yachandra VK, Christou G (2012) Synthetic model of the asymmetric [Mn3CaO4] cubane core of the oxygen-evolving complex of photosystem II. Proc Natl Acad Sci USA 109(7):2257–2262
Ono T, Inoue Y (1988) Discrete extraction of the Ca atom functional for O2 evolution in higher plant photosystem II by a simple low pH treatment. FEBS Lett 227(2):147–152
Ono T, Rompel A, Mino H, Chiba N (2001) Ca2+ function in photosynthetic oxygen evolution studied by alkali metal cations substitution. Biophys J 81(4):1831–1840
Pitari F, Bovi D, Narzi D, Guidoni L (2015) Characterization of the Sr2+- and Cd2+-substituted oxygen-evolving complex of photosystem II by quantum mechanics/molecular mechanics calculations. Biochemistry 54(38):5959–5968. https://doi.org/10.1021/acs.biochem.5b00797
Saito K, Ishikita H (2014) Influence of the Ca2+ ion on the Mn4Ca conformation and the H-bond network arrangement in photosystem II. Biochim Biophys Acta 1837(1):159–166
Saito K, Shen J-R, Ishida T, Ishikita H (2011) Short hydrogen-bond between redox-active tyrosine YZ and D1-His190 in the photosystem II crystal structure. Biochemistry 50:9836–9844
Saito K, Mandal M, Ishikita H (2020a) Energetics of ionized water molecules in the H-bond network near the Ca2+ and Cl− binding sites in photosystem II. Biochemistry 59(35):3216–3224. https://doi.org/10.1021/acs.biochem.0c00177
Saito K, Mandal M, Ishikita H (2020b) Redox potentials along the redox-active low-barrier H-bonds in electron transfer pathways. Phys Chem Chem Phys. https://doi.org/10.1039/d0cp04265j
Saito K, Nakagawa M, Ishikita H (2020c) pKa of the ligand water molecules in the oxygen-evolving Mn4CaO5 cluster in photosystem II. Commun Chem 3(1):89. https://doi.org/10.1038/s42004-020-00336-7
Schmidt am Busch M, Knapp E-W (2005) One-electron reduction potential for oxygen- and sulfur-centered organic radicals in protic and aprotic solvents. J Am Chem Soc 127:15730–15737
Shannon RD (1976) Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A Cryst Phys Diffr Theor Gen Crystallogr 32:751–767
Shen JR (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48. https://doi.org/10.1146/annurev-arplant-050312-120129
Siegbahn PEM (2014) Water oxidation energy diagrams for photosystem II for different protonation states, and the effect of removing calcium. Phys Chem Chem Phys 16(24):11893–11900. https://doi.org/10.1039/c3cp55329a
Siegbahn PE (2017) Water oxidation by PSII—a quantum chemical approach. In: Wikström M (ed) Mechanisms of primary energy transduction in biology. The Royal Society of Chemistry, London, pp 273–295
Tsui EY, Agapie T (2013) Reduction potentials of heterometallic manganese-oxido cubane complexes modulated by redox-inactive metals. Proc Natl Acad Sci USA 110(25):10084–10088
Tsui EY, Tran R, Yano J, Agapie T (2013) Redox-inactive metals modulate the reduction potential in heterometallic manganese-oxido clusters. Nat Chem 5(4):293–299
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473(7345):55–65. https://doi.org/10.1038/nature09913
Vacek G, Perry JK, Langlois JM (1999) Advanced initial-guess algorithm for self-consistent-field calculations on organometallic systems. Chem Phys Lett 310:189–194
Yachandra VK, Yano J (2011) Calcium in the oxygen-evolving complex: structural and mechanistic role determined by X-ray spectroscopy. J Photochem Photobiol B 104(1–2):51–59
Yamaguchi K, Yamanaka S, Isobe H, Saito T, Kanda K, Umena Y, Kawakami K, Shen J-R, Kamiya N, Okumura M, Nakamura H, Shoji M, Yoshioka Y (2013) The nature of chemical bonds of the CaMn4O5 cluster in oxygen evolving complex of photosystem II: Jahn-Teller distortion and its suppression by Ca doping in cubane structures. Int J Quantum Chem 113:453–473
Zhang G, Musgrave CB (2007) Comparison of DFT methods for molecular orbital eigenvalue calculations. J Phys Chem A 111(8):1554–1561. https://doi.org/10.1021/jp061633o
Zhang CX, Chen CH, Dong HX, Shen JR, Dau H, Zhao JQ (2015) A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis. Science 348(6235):690–693
Zimmermann JL, Rutherford AW (1986) Electron paramagnetic resonance properties of the S2 state of the oxygen-evolving complex of photosystem II. Biochemistry 25(16):4609–4615. https://doi.org/10.1021/bi00364a023
Funding
This study was supported by JST CREST (JPMJCR1656 to H.I.), JSPS KAKENHI (18H05155, 18H01937, 20H03217, and 20H05090 to H.I.; 18H01186 to K.S.; and 16H06560 to K.S.), and the Interdisciplinary Computational Science Program in CCS, University of Tsukuba (K.S.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saito, K., Nakagawa, M., Mandal, M. et al. Role of redox-inactive metals in controlling the redox potential of heterometallic manganese–oxido clusters. Photosynth Res 148, 153–159 (2021). https://doi.org/10.1007/s11120-021-00846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-021-00846-y