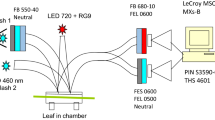
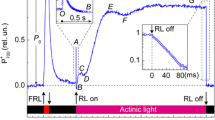
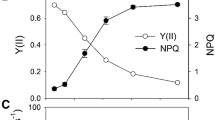
Abstract
The present work was aimed to explain the recently reported higher O2-dependent electron flow capacity in gymnosperms than in angiosperms and to search for other differences in the electron transport processes by simultaneous characterization of the relative capacities of pseudocyclic (direct or Flavodiiron proteins (Flv)-mediated O2-reduction, Mehler(-like) reactions) and cyclic electron flows around photosystem I (CEF-PSI). To this end, a comparative multicomponent analysis was performed on the fluorescence decay curves of dark-adapted leaves after illumination with a 1-s saturating light pulse. In both gymnosperms and angiosperms, two or three exponential decay components were resolved: fast (t 1/21 ~ 170–260 ms), middle (~1.0–2.3 s), and slow (>4.2 s). The sensitivity of the decay parameters (amplitudes A1–3, halftimes t 1/2 1–3) to the alternative electron flows was assessed using Arabidopsis pgr5 and ndhM mutants, defective in CEF-PSI, Synechocystis sp. PCC 6803 Δflv1 mutant, defective in Flv-mediated O2-photoreduction, different O2 concentrations, and methyl viologen treatment. A1 reflected the part of electrons involved in linear and O2-photoreduction pathways after PSI. The middle component appeared in pgr5 (but not in ndhM), in gymnosperms under low O2, and in Δflv1, and reflected limitations at the PSI acceptor side. The slow component was sensitive to CEF-PSI. The comparison of decay parameters provided evidence that Flv mediate O2-photoreduction in gymnosperms, which explains their higher O2-dependent electron flow capacity. The concomitant quantification of relative electrons branching in O2-photoreduction and CEF-PSI pathways under the applied non-steady-state photosynthetic conditions reveals that CEF-PSI capacity significantly exceeds that of O2-photoreduction in angiosperms while the opposite occurs in gymnosperms.




Similar content being viewed by others
Abbreviations
- CEF-PSI:
-
Cyclic electron flows around photosystem I
- Chl:
-
Chlorophyll
- Flv:
-
Flavodiiron proteins
- Fd:
-
Ferredoxin
- FR:
-
Far-red
- IRF:
-
Instrument response function
- LEF:
-
Linear electron flow
- MV:
-
Methyl viologen
- NDH:
-
NADH dehydrogenase-like complex
- PGR5:
-
Proton gradient regulation 5
- PGRL1:
-
Pgr5-like photosynthetic phenotype 1
- PQ:
-
Plastoquinone
- PS:
-
Photosystem
- QA(B) :
-
The primary (secondary) quinone electron acceptor in PSII
- SP:
-
Saturating pulse
References
Allahverdiyeva Y, Ermakova M, Eisenhut M, Zhang P, Richaud P, Hagemann M, Cournac L, Aro EM (2011) Interplay between Flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J Biol Chem 286:24007–24014. doi:10.1074/jbc.M111.223289
Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110:4111–4116. doi:10.1073/pnas.1221194110
Allahverdiyeva Y, Isojärvi J, Zhang P, Aro EM (2015) Cyanobacterial oxygenic photosynthesis is protected by Flavodiiron proteins. Life 5:716–743. doi:10.3390/life5010716
Asada K (2000) The water–water cycle as alternative photon and electron sinks. Philos Trans R Soc B 355:1419–1431. doi:10.1098/rstb.2000.0703
Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos Trans R Soc B 355:1433–1446. doi:10.1098/rstb.2000.0704
Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15:330–336. doi:10.1016/j.tplants.2010.03.006
Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W (2009) On-line mass spectrometry: membrane inlet sampling. Photosynth Res 102:511–522. doi:10.1007/s11120-009-9474-70
Bukhov N, Egorova E, Krendeleva T, Rubin A, Wiese C, Heber U (2001) Relaxation of variable chlorophyll fluorescence after illumination of dark-adapted barley leaves as influenced by the redox states of electron carriers. Photosynth Res 70:155–166. doi:10.1023/A:1017950307360
Christenhusz MJM, Byng JW (2016) The number of known plants species in the world and its annual increase. Phytotaxa 261:201–217. doi:10.11646/phytotaxa.261.3.1
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285. doi:10.1016/j.cell.2007.12.028
Deák Z, Sass L, Kiss É, Vass I (2014) Characterization of wave phenomena in the relaxation of flash-induced chlorophyll fluorescence yield in cyanobacteria. Biochim Biophys Acta 1837:1522–1532. doi:10.1016/j.bbabio.2014.01.003
Desai TS, Rane SS, Tatake VG (1983) Identification of far-red-induced relative increase in the decay of delayed light emission from photosynthetic membranes with thermoluminescence peak V appearing at 321 K. Biochim Biophys Acta 724:485–489. doi:10.1016/0005-2728(83)90109-3
Ducruet JM (2003) Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. J Exp Bot 54:2419–2430. doi:10.1093/jxb/erg268
Fan DY, Fitzpatrick D, Oguchi R, Ma W, Kou J, Chow WS (2016) Obstacles in the quantification of the cyclic electron flux around photosystem I in leaves of C3 plants. Photosynth Res 129:239–251. doi:10.1007/s11120-016-0223-4
Finazzi G, Johnson GN (2016) Cyclic electron flow: facts and hypotheses. Photosynth Res 129:227–230. doi:10.1007/s11120-016-0306-2
Fischer WW, Hemp J, Valentine JS (2016) How did life survive Earth’s great oxygenation? Curr Opin Chem Biol 31:166–178. doi:10.1016/j.cbpa.2016.03.013
Fisher N, Kramer DM (2014) Non-photochemical reduction of thylakoid photosynthetic redox carriers in vitro: relevance to cyclic electron flow around photosystem I? Biochim Biophys Acta 1837:1944–1954. doi:10.1016/j.bbabio.2014.09.005
Golbeck JH, Cornelius JM (1986) Photosystem I charge separation in the absence of centers A and B. I. Optical characterization of centre A2 and evidence for its association with a 64 kDa peptide. Biochim Biophys Acta 849:16–24. doi:10.1016/0005-2728(86)90091-5
Gotoh E, Kobayashi Y, Tsuyama M (2010) The post-illumination chlorophyll fluorescence transient indicates the RuBP regeneration limitation of photosynthesis in low light in Arabidopsis. FEBS Lett 584:3061–3064. doi:10.1016/j.febslet.2010.05.039
Hald S, Nandha B, Gallois P, Johnson GN (2008) Feedback regulation of photosynthetic electron transport by NADP(H) redox poise. Biochim Biophys Acta 1777:433–440. doi:10.1016/j.bbabio.2008.02.007
Havaux M, Rumeau D, Ducruet JM (2005) Probing the FQR and NDH activities involved in cyclic electron transport around Photosystem I by the ‘afterglow’ luminescence. Biochim Biophys Acta 1709:203–213. doi:10.1016/j.bbabio.2005.07.010
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R Ohad I, Kaplan A (2003) Genes encoding A-Type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13:230–235. doi:10.1016/S0960-9822(03)00046-0
Ilík P, Pavlovič A, Kouřil R, Alboresi A, Morosinotto T, Allahverdiyeva Y, Aro EM, Yamamoto H, Shikanai T (2017) Alternative electron transport mediated by Flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol 214:967–972. doi:10.1111/nph.14536
Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464:1210–1213. doi:10.1038/nature08885
Jia H, Oguchi R, Hope AB, Barber J, Chow WS (2008) Differential effects of severe water stress on linear and cyclic electron fluxes through photosystem I in spinach leaf discs in CO2-enriched air. Planta 228:803–812. doi:10.1007/s00425-008-0783-4
Katsumata M, Takeuchi A, Kazumura K, Koike T (2008) New feature of delayed luminescence: preillumination-induced concavity and convexity in delayed luminescence decay curve in the green alga Pseudokirchneriella subcapitata. J Photochem Photobiol B 90:152–162. doi:10.1016/j.jphotobiol.2007.12.005
Kono M, Noguchi K, Terashima I (2014) Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol 55:990–1004. doi:10.1093/pcp/pcu033
Laisk A, Eichelmann H, Oja V, Peterson RB (2005) Control of cytochrome b 6 f at low and high light intensity and cyclic electron transport in leaves. Biochim Biophys Acta 1708:79–90. doi:10.1016/j.bbabio.2005.01.007
Laisk A, Eichelmann H, Oja V, Peterson RB (2015) Oxidation of plastohydroquinone by photosystem II and by dioxygen in leaves. Biochim Biophys Acta 1847:565–575. doi:10.1016/j.bbabio.2015.03.003
Ma G, Mincu N, Lesage F, Gallant P, McIntosh L (2005) System IRF impact on fluorescence lifetime fitting in turbid medium. Proc SPIE 5699:263–273. doi:10.1117/12.589338
Mehler AH (1951) Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch Biochem Biophys 33:65–77. doi:10.1016/0003-9861(51)90082-3
Miyake C (2010) Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963. doi:10.1093/pcp/pcq173
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371. doi:10.1016/S0092-8674(02)00867-X
Mustila H, Paananen P, Battchikova N, Santana-Sánchez A, Muth-Pawlak D, Hagemann M, Aro E-M, Allahverdiyeva Y (2016) The Flavodiiron protein Flv3 functions as a homo-oligomer during stress acclimation and is distinct from the Flv1/Flv3 hetero-oligomer specific to the O2 photoreduction pathway. Plant Cell Physiol 57:1468–1483. doi:10.1093/pcp/pcw047
Okegawa Y, Long TA, Iwano M, Takayama S, Kobayashi Y, Covert SF, Shikanai T (2007) A balanced PGR5 level is required for chloroplast development and optimum operation of cyclic electron transport around photosystem I. Plant Cell Physiol 48:1462–1471. doi:10.1093/pcp/pcm116
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198. doi:10.1016/S1369-5266(02)00259-5
Peltier G, Tolleter D, Billon E, Cournac L (2010) Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth Res 106:19–31. doi:10.1007/s11120-010-9575-3
Renger G, Eckert HJ, Bergmann A, Bernarding J, Liu B, Napiwotzki A, Reifarth F, Eichler HJ (1995) Fluorescence and spectroscopic studies of exciton trapping and electron transfer in photosystem II of higher plants. Funct Plant Biol 22:167–181. doi:10.1071/PP9950167
Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G (2005) New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 17:219–232. doi:10.1105/tpc.104.028282
Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30:1041–1051. doi:10.1111/j.1365-3040.2007.01675.x
Ruuska SA, Badger MR, Andrews TJ, von Caemmerer S (2000) Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. J Exp Bot 51:357–368. doi:10.1093/jexbot/51.suppl_1.357
Schreiber U, Endo T, Mi H, Asada K (1995) Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol 36:873–882. doi:10.1093/oxfordjournals.pcp.a078833
Schuurmans RM, van Alphen P, Schuurmans JM, Matthijs HCP, Hellingwerf KJ (2015) Comparison of the photosynthetic yield of cyanobacteria and green algae: different methods give different answers. PLoS ONE 10:e0139061. doi:10.1371/journal.pone.0139061
Shikanai T, Yamamoto H (2017) Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol Plant 10:20–29.
Shimakawa G, Shaku K, Nishi A, Hayashi R, Yamamoto H, Sakamoto K Makino A, Miyake C (2015) FLAVODIIRON2 and FLAVODIIRON4 proteins mediate an oxygen-dependent alternative electron flow in Synechocystis sp. PCC 6803 under CO2-limited conditions. Plant Physiol 167:472–480. doi:10.1104/pp.114.249987
Shirao M, Kuroki S, Kaneko K, Kinjo Y, Tsuyama M, Förster B, Takahashi S, Badger MR (2013) Gymnosperms have increased capacity for electron leakage to oxygen (Mehler and PTOX reactions) in photosynthesis compared with angiosperms. Plant Cell Physiol 54:1152–1163. doi:10.1093/pcp/pct066
Stirbet A, Govindjee (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J–I–P rise. Photosynth Res 113:15–61. doi:10.1007/s11120-012-9754-5
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation. Taylor and Francis, London, pp 443–480
Takagi D, Ishizaki K, Hanawa H, Mabuchi T, Shimakawa G, Yamamoto H, Miyake C (2017) Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol Plantarum. doi:10.1111/ppl.12562
Takahashi Y, Katoh S (1984) Triplet states in a photosystem I reaction center complex. Inhibition of radical pair recombination by bipyridinium dyes and naphthoquinones. Plant Cell Physiol 25:785–794. doi:10.1093/oxfordjournals.pcp.a076773
Teuchner K (1979) Entwicklung eines Laser-Fluoreszenzspectralphotometers und seine Anwendung in der Nanosekundenspektroskopie organischer Moleküle, Dissertation, Humboldt University of Berlin
Tsuyama M, Kobayashi Y (2009) Reduction of the primary donor P700 of photosystem I during steady-state photosynthesis under low light in Arabidopsis. Photosynth Res 99:37–47. doi:10.1007/s11120-008-9379-x
Vass I, Kirilovsky D, Etienne AL (1999) UV-B radiation-induced donor- and acceptor-side modifications of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. BioChemistry 38:12786–12794. doi:10.1021/bi991094w
Yamamoto H, Kato H, Shinzaki Y, Horiguchi S, Shikanai T, Hase T, Endo T, Nishioka M, Makino A, Tomizawa K, Miyake C (2006) Ferredoxin limits cyclic electron flow around PSI (CEF-PSI) in higher plants—Stimulation of CEF-PSI enhances non-photochemical quenching of Chl fluorescence in transplastomic Tobacco. Plant Cell Physiol 47:1355–1371. doi:10.1093/pcp/pcl005
Yamamoto H, Takahashi S, Badger MR, Shikanai T (2016) Artificial remodeling of alternative electron flow by Flavodiiron proteins in Arabidopsis. Nature Plants 2:16012. doi:10.1038/nplants.2016.12
Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67:81–106. doi:10.1146/annurev-arplant-043015-112002
Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4:e5331. doi:10.1371/journal.pone.0005331
Acknowledgements
We thank the Kyushu University Forest for collection of the materials. R. V. thanks the Bulgarian Academy of Sciences for their support.
Funding
This work was supported by a grant-in-aid for scientific research from JSPS (No. 26450200) and by the JSPS Invitation Fellowships in Japan (to R. V.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noridomi, M., Nakamura, S., Tsuyama, M. et al. Opposite domination of cyclic and pseudocyclic electron flows in short-illuminated dark-adapted leaves of angiosperms and gymnosperms. Photosynth Res 134, 149–164 (2017). https://doi.org/10.1007/s11120-017-0419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0419-2