Abstract
Ocean acidification, one of the great global environmental issues at present, is expected to result in serious damage on marine calcareous organisms such as corals and calcifying algae, which potentially release huge amounts of CO2 from the ocean to the atmosphere. The coccolithophore, Emiliania huxleyi (Haptophyceae), which frequently produces blooms, has greatly contributed to the biological CO2 pump. This study was aimed at analyzing effects of how E. huxleyi responds to acidification. Acidification was performed by two methods, namely by just adding HCl under bubbling ordinary air at 8.2–8.4, 7.6–7.8 and 7.1–7.3 (acidification by HCl) and by bubbling with ordinary air or with increased CO2 concentration such as 406, 816 and 1,192 ppm that maintained pH of the medium at 8.0–8.3, 7.6–7.9 and 7.5–7.7 (acidification by CO2 enrichment). As a result, cell growth and cellular calcification of E. huxleyi were strongly damaged by acidification by HCl, but not by acidification by CO2 enrichment. The activities of photosystems such as F v/F m and ϕPSII were not affected by any acidification conditions while photosynthetic O2 evolution was slightly stimulated. A 45Ca-radiotracer experiment revealed that Ca2+-uptake was strongly suppressed by acidification with HCl. This suppression recovered after increasing the dissolved inorganic carbon (DIC) concentration and further stimulated by an additional increase in DIC concentration. The production of storage and coccolith polysaccharides was increased by acidification by HCl and also highly stimulated by acidification with CO2 enrichment. The present study clearly showed that the coccolithophore, E. huxleyi, has an ability to respond positively to acidification with CO2 enrichment, but not just acidification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increase in atmospheric CO2 concentration is now recognized to have increased ocean acidification (Orr et al. 2005; Zeebe et al. 2008). Oceanic pH has already decreased 0.1 U ever since the industrial revolution in the eighteenth century, and it is speculated to decrease 0.5 U further by the end of the twenty-first century according to IPCC scenario. The pH of the surface ocean is estimated to decrease by 0.3–0.5 and 0.7–0.77 U relative to the present level by 2,100 (pH 7.6–7.9) and 2,300 (pH 7.33–7.5), respectively (Caldeira and Wickett 2003; Ross et al. 2011). Such rapid ocean acidification is believed to have negative influences on marine organism with calcifying organisms as prime targets for strong damage by acidification (Feely et al. 2004), e.g., the bleaching and reduction of coral reefs (Gattuso et al. 1998; Kleypas et al. 1999; Hoegh-Guldberg et al. 2007; Anthony et al. 2008; Kuffner et al. 2008; Veron et al. 2009). In addition, the shell of gastropod, Littorina littorea, and foraminifera are shown to lose hardness by acidification (Bibby et al. 2007; Bijma et al. 2002). The fertilization rate of sea urchin, Psammechinus miliaris, declined with acidification (Miles et al. 2007). Such influence of oceanic acidification is expected to affect the entire ecosystem and damage the oceanic environment. However, even under such circumstances, actual events caused by acidification have not been investigated thoroughly in individual organisms (Richier et al. 2010).
In particular, a marine calcifying haptophycean alga, Emiliania huxleyi, is affected by ocean acidification (Iglesias-Rodriguez et al. 2008; Langer et al. 2006; Riebesell et al. 2000) because E. huxleyi forms cell-covering, calcium carbonate crystals, called coccoliths. The alga is known to distribute widely in the world ocean, fix a large amount of carbon, produce a huge biomass and carry carbon from sea surface to the sediment by the biological CO2 pump (Liu et al. 2009). Therefore, E. huxleyi can be said to have played very important roles in the global carbon cycle. Riebesell et al. (2000) reported a reduction in calcification by E. huxleyi under future scenarios on ocean acidification. However, Iglesias-Rodriguez et al. (2008) observed enhanced calcification under elevated pCO2 in E. huxleyi. Hoppe et al. (2011) reported that E. huxleyi shows identical responses to elevated pCO2 in total alkalinity (TA) and dissolved inorganic carbon (DIC) manipulations. They also showed that different experimental protocols (e.g., continuous bubbling versus pre-bubbled) can lead to change in growth rates and other ecophysiological parameters.
The coccolithophore E. huxleyi has influenced the global climate for over 200 million years and therefore is thought to have played critical roles in the global carbon cycle. Even in the present ocean, the algae are widely distributed globally and it is well known that they fix a large amount of carbon, produce a huge biomass and carry carbon from the sea surface to the sediment by the biological CO2 pump (Liu et al. 2009). Recently, Read et al. (2013) reported the first haptophyte reference genome, from E. huxleyi CCMP1516, and sequences from 13 additional isolates. It revealed that a pan genome (core genes plus genes distributed variably between strains) is probably supported by an atypical compliment of respective sequences in the genome. They assumed that such a wide variation of genomes in E. huxleyi seems to be the reason for forming large-scale episodic blooms under a wide variety of environmental conditions.
In this study, we investigated the physiological response of the coccolithophore E. huxleyi to acidification by experimental acid enrichment (acidification by HCl) and by ventilation of air with elevated concentration of CO2 (acidification by CO2 enrichment). These conditions are not exactly the same as the ocean acidification conditions being observed in the ocean, but will give important information on how E. huxleyi will respond to acidification. Finally, we clearly show that just acidification caused by HCl is disadvantageous to E. huxleyi, but acidification by CO2 enrichment induced positive influence on photosynthesis and calcification of the organism. This study also proved clearly that the suppression of intracellular calcification by acidification in the coccolithophore is due to the reduction of HCO3 − supply, which is the substrate for intracellular calcium carbonate crystal production, because the suppression of calcification recovered following additional supply of bicarbonate ions.
Materials and methods
Material and culture conditions
The strain (NIES 837) of the coccolithophore E. huxleyi (Lohmann) Hay and Mohler (Haptophyta) used in this study was collected by Dr. I. Inouye in the South Pacific Ocean in 1990 and has been maintained at 20 °C under 16-h light/8-h dark regime in our laboratory. Cells were maintained in natural seawater for stock culture. For experimental culture, the medium used was an artificial seawater (Marine Art SF-1; produced by Tomita Seiyaku Co., Ltd., Tokushima, Japan, distributed by Osaka Yakken Co., Ltd., Osaka, Japan) enriched with a micronutrient mixture of the Erd-Schreiber’s medium (ESM) in which soil extracts are replaced with 10 nM sodium selenite according to Danbara and Shiraiwa (1999). ESM enrichment contains 28.7 μM (final concentration in the medium) K2HPO4, but not in the Marine Art SF. In all acidification experiments, cells were grown in the artificial seawater containing EMS medium (MA/ESM medium) under constant illumination at 100 μmol photons m−2 s−1 and 20 °C (standard condition). To avoid large changes in the pH of the medium during culture, both HEPES and Tris-buffer (final concentration, 10 mM each) were added to the medium by considering those buffers’ buffering ability and pKa values.
Bubbling cultures with air and air containing elevated concentration of CO2
Tanks containing air with elevated concentrations of CO2, namely 406, 816 and 1192 ppm, were purchased from the company, Suzuki Shokan Ltd., Tsukuba, Japan. First, those gasses were bubbled through MA/EMS medium containing HEPES- and Tris-buffers (10 mM each) for 15 h as pre-bubbling for attaining equilibrium of CO2 between the bubbled gasses and the medium. The concentrations of respective DIC species in the medium shown in Fig. 1 and 6 were calculated according to Leuker et al. (2000) and CO2SYS, respectively. On the other hand, algal cells were grown separately with air in the MA/ESM medium under constant illumination at 100 μmol m−2 s−1 and 20 °C for 3 days. And then, an aliquot of the algal suspension was transferred to the previously prepared medium of which pH and pCO2 were already set by adding HCl or bubbling of air containing elevated CO2, as described above.
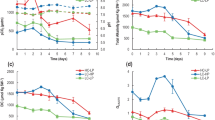
Effect of the acidification by HCl (a–e) and the ocean acidification conditions by elevating pCO2 (f–j) on the cell growth of the coccolithophore E. huxleyi. Before experiments, all cells had been grown at pH 8.2 under the bubbling of air containing 400 ppm CO2. Temperature was 20 °C. a, f, Change in turbidity; b, g change in cell number; c, h H in the medium. Initial pHs were set at 8.2 in a (closed circles), 7.7 in closed squares and 7.2 in closed triangles by HCl (a–c) and at 7.9 in closed circles, 7.6 in closed squares and 7.5 closed triangles by elevating pCO2 (f–h). d, i Specific growth rates (μ) calculated on the basis of cell number; e, j inorganic carbon concentrations in the medium at each pH and the elevated pCO2 concentration at 1 day. CO2 concentration was set at 15 μmol L−1 in all the conditions (right column). Solid (left) and stripe (middle) columns indicate total DIC and HCO3 − concentrations, respectively. DIC, bicarbonate and CO2 concentrations were calculated by a kind help of Dr. Midorikawa according to Leuker et al. (2000)
Determination of the specific growth rate and microscopic observation
Cell turbidity of the culture was determined by measuring OD750 using a spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan). Cell number was determined under a microscope by counting cells on a ready-made glass slide using a microscopic camera system and a counter. The specific growth rate (μ) was calculated by the equation of \( \mu = { \ln }\left[ {{{\left( {m_{{t_{1} }} - m_{{t_{ 1} }} } \right)} \mathord{\left/ {\vphantom {{\left( {m_{{t_{1} }} - m_{{t_{ 1} }} } \right)} {(t_{ 2} - t_{ 1} )}}} \right. \kern-0pt} {(t_{ 2} - t_{ 1} )}}} \right], \) where m x represents cell number at arbitrary time t 1 and t 2 (t 2 > t 1) during the logarithmic growth phase. Coccoliths covering cells were visualized under polarized light by a microscope (Olympus Ltd., Tokyo, Japan) equipped with a fluorescence microscope digital camera (Keyence, Osaka, Japan).
Determination of photosynthetic activity
The algal cells were harvested from the culture and then centrifuged (700×g for 10 min at 15 °C) to obtain a cell pellet. After suspending cells in adequate buffers, photosynthetic O2 evolution activity was determined by a Clark-type oxygen electrode (Rank Brothers Co., Ltd., UK). The light intensity and temperature were maintained at 270 μ mol photons m−2 s−1 and 25 °C, respectively. The light source was a white LED lamp (Model HLV-24SW-3W, CCS, Kyoto, Japan).
Determination of photosystem activity expressed with chlorophyll fluorescence parameters
Photosystems of E. huxleyi were characterized by the chlorophyll fluorescence method. First, chlorophyll concentration of cells was determined in 90 % methanol extracts by a spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan) according to published procedures (Jeffrey 1972). Then algal concentration was adjusted to 5.0 μg Chl mL−1 in the MA/ESM medium (final phosphate concentration, 28.7 μM) at different pHs (7.2–8.2) for measurements. Photosystem activity was determined using a FluorCam (MF 701, Photon Systems Instruments, Bruno, Czech Republic), and the parameters of F v/F m and ϕPSII were calculated by manufactured software attached to the apparatus. The duration and intensity of excitation light were 20 min and 100 μmol photons m−2 s−1, respectively, and of measured saturated pulsed light were 800 ms and 2,000 μmol photons m−2 s−1, respectively. Dissolved inorganic carbon (DIC) concentration was 2 mM, which was equilibrated with atmospheric CO2 concentration at pH 8.2.
45Ca uptake assay
Effect of pH on calcification was tested by a radiotracer method. The cells were harvested by centrifugation (700×g for 10 min at 15 °C) and re-suspended into the fresh experimental culture medium. The pH of the medium was adjusted at either pH 7.2, 7.7 or 8.2 by adding an aliquot of 0.2 N HCl. An aliquot of 45CaCl2 solution (Perkin-Elmer, Inc., Waltham, MA, USA) was directly injected into algal cell culture. Final concentration and the specific radioactivity of 45Ca in the medium were 10 mM and 20 MBq mmol−1, respectively. The algal suspension was continuously bubbled with ordinary air at a speed of 100 mL min−1. Subsequent experimental procedure for the determination of 45Ca uptake activity was according to the method of Kayano and Shiraiwa (2009). According to our previous results in the same strain of E. huxleyi, more than 95 % of calcium absorbed by cells is utilized for calcification (Satoh et al. 2009) and therefore the measurement of 45Ca-uptake could be used as a good parameter for calcification activity in this study.
Assays
As the coccolith contains the coccolith polysaccharides, which are acid polysaccharides composed of uronic acids (Kayano and Shiraiwa 2009), uronic acid content was used as a parameter of acid polysaccharide (AP) production. The carbazole–H2SO4 assay (Bitter and Muir 1962) was used for the determination of uronic acid content using 0–90 μg mL−1 glucuronic acid (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) as a standard for calibration.
The amount of total polysaccharides (TP) included both AP and neutral polysaccharides (NP) composed of reducing sugars. TP was estimated as total sugars using a phenol–H2SO4 assay using 0–90 μg mL−1 glucose as a standard for calibration (Hodge and Hofreiter 1962). Then, the amount of NP was calculated by TP − AP.
The polysaccharides were analyzed by SDS-PAGE on a 15 % acrylamide gel. After electrophoresis, the gels were stained with Stains-all (Applichem GmbH, A1400.0001, Cheshire, USA) and Alcian blue (Sigma-Aldrich, A5268-10G, Missouri, USA) for determining TP and AP, respectively. The quantitative analysis of the protein used BIO-RAD DC protein Assay kit (Bio-Rad Laboratories AB, 500-0111, Oslo, Norway) using albumin as a standard for calibration.
Results
Effect of acidification on the growth of E. huxleyi
The growth curve of E. huxleyi determined by cell number and turbidity showed clear suppression by acidification with HCl under the aeration of ordinary air (Fig. 1a, b). The pH values of the medium in three cultures were maintained nearly constant with slight increases from 8.2 to 8.4 (8.2 for first 4 days), 7.7 to 7.9 (7.7 for first 4 days) and 7.2 to 7.3 (ca. 7.2 for first 4 days) during 7 days (Fig. 1c). The pH values for first 4 days were used to express culture conditions in the text. The specific growth rate (μ) decreased by acidification ca. 30 and 60 % at pH 7.7 and 7.2, respectively, in comparison with that at pH 8.2 (Fig. 1d). Cell growth at pH 7.2 was rapidly and strongly suppressed in a day, and then, cells were destroyed (Fig. 1a, b). The concentrations of total DIC and bicarbonate ions at pH 7.7 and 7.2 cultures were 75 and 90 % lower than that at pH 8.2 culture (Fig. 1e). As dissolved CO2 (dCO2) concentration in the medium is maintained as a constant according to the Henry’s law under bubbling of air, the suppression of growth at low pHs should be due to the combination of acidification effect and the decrease in HCO3 − concentrations equilibrated with air (Fig. 1e).
On the other hand, the growth of E. huxleyi was almost the same among different CO2 concentrations and pHs when acidification was performed by the bubbling of air containing elevated CO2 concentration such as 406, 816 and 1,192 ppm (acidification by CO2 enrichment) (Fig. 1f, g). During the culture for 7 d, the pH of the medium was maintained at 8.0–8.3, 7.6–7.9 and 7.5–7.7 by the bubbling of air containing 406, 816 and 1,192 ppm CO2, respectively (Fig. 1h). The specific growth rate (μ) was slightly higher ca. 15 and 25 % at 816 and 1,192 ppm CO2, respectively, in comparison with that at 406 ppm CO2 (Fig. 1i). Under such conditions, total DIC and bicarbonate concentrations were almost the same among the three different CO2 conditions resulting in different pHs (Fig. 1h) where dCO2 concentrations were increased according to the elevation of CO2 concentration (Fig. 1j).
Effect of acidification on photosynthetic activity in E. huxleyi
The photosynthetic O2 evolution activity was not affected when pH of the medium decreased (Fig. 2a–c, g), suggesting that photosynthetic machinery was hardly damaged by acidification with HCl. However, photosynthetic activity changed during the 7-day experiment at every pH tested. Although the reason is unclear yet, it maybe associated with the depletion of inorganic phosphate from the medium during growth, according to our previous study (Satoh et al. 2009). Photosynthetic O2 evolution activity was slightly higher at higher CO2 concentration when compared among the 406, 816 and 1,192 ppm CO2 experiments, where pH values were maintained at 7.9–8.3, 7.6–7.9 and 7.5–7.7 (Fig. 2d–f, g). The highest average value of photosynthetic O2 evolution was 150 μmol (mg Chl)−1 h−1 at pH 7.5–7.7, which was attained by the bubbling of air containing 1,192 ppm CO2 (Fig. 2g). These results show that the response of photosynthetic activity to pH change was almost the same, irrespective of the method of how pH was decreased, namely by adding HCl or bubbling air with elevated CO2.
Effect of the acidification by HCl (a–c) and the ocean acidification conditions by elevating pCO2 (d–f) on the changes in photosynthetic O2 evolution activity of the coccolithophore E. huxleyi. Experimental conditions for acclimation (indicated in the figure) were same as shown in Fig. 1. The rate of photosynthetic O2 evolution was determined using a Clark-type O2 electrode at the light intensity of 270 μmol photons m−2 s−1 and 25 °C which are the optimum conditions. The values are average of three experiments (n = 3)
The activities of the photosystems were determined by measuring F v/F m, which reflects the state of photosystem II (Demmig and Bjorkman 1987) and ϕPSII, which is an index of the electron transport activity of the whole photosystem (Genty et al. 1989). The results indicate that the photosystem parameters determined were not changed, namely almost the same, during the 6-day experiment between pH 7.7 and 8.2 (Fig. 3a, b). On the other hand, F v/F m decreased similarly after 3 days under all tested CO2 conditions when pH was set by the bubbling of air containing various CO2 (Fig. 3c, e). Under the same conditions, ϕPSII was maintained almost constant for 6 days after rapidly decreasing during the first 2 days at 406 and 816 ppm CO2 conditions (Fig. 3c, d). There are no data on 0 day since the measurement of photosystem activities in the CO2 ventilation was begun after 1 day.
Effect of the acidification by HCl (a, b) and the ocean acidification conditions by elevating pCO2 (c–e) on the changes in the parameters of photosystem activity such as F v/F m and ϕPSII during growth of the coccolithophore E. huxleyi. The chlorophyll fluorescence parameters were determined by Fluorcam, as described in “Materials and methods.” Solid line (circles), F v/F m; dotted line (square), ϕPSII. Error bars ±SD (n = 3)
Effect of acidification on coccolith production and calcification by E. huxleyi
Polarized light microscopic observations clearly showed that coccolith production was strongly suppressed when acidification was performed by HCl from 8.2 to pH 7.7 and 7.2 (Fig. 4a). In contrast, coccolith production was strongly stimulated and accompanied by an increase in cell size when pH was maintained at 8.0–8.3, 7.6–7.9 and 7.5–7.7 by the bubbling air containing various CO2 concentrations with 406, 816 and 1,192 ppm, respectively (Fig. 4b).
Effect of the acidification by HCl (a) and the ocean acidification conditions by elevating pCO2 (b) on the microscopic images for coccolith production and cell size of the coccolithophore E. huxleyi. The cells were grown for 12 days under each condition. Experimental conditions for acclimation (indicated in the figure) were same as shown in Fig. 1
E. huxleyi needs to incorporate and accumulate calcium and bicarbonate ion as substrates for intracellular coccolith production into the coccolith vesicles within the coccolithophore cells. The rate of 45Ca-incorporation activity was strongly suppressed to 22 and 7 % at 7.7 and 7.2, respectively, in comparison with that of pH 8.2 when pH values were set by acidification with HCl under continuous bubbling of ordinary air (Fig. 5). When the concentration of CO2 dissolved in the solution is equilibrated with atmospheric air, bicarbonate concentration is calculated to be almost the same between pHs 8.2 and 7.7, but carbonate concentration is much higher at pH 8.2 than 7.7 (Fig. 6d). These data clearly show that 45Ca-incorporation into cells was greatly diminished by acidification with HCl, although the concentration of bicarbonate, the substrate to be absorbed by cells for intracellular calcification (Sekino and Shiraiwa 1994), was the same at both pHs.
Effect of the acidification by HCl on 45Ca-uptake by the coccolithophore E. huxleyi. In order to stimulate coccolith production, cells grown for 12 days were transferred to the orthophosphate-free medium for the radiotracer experiments. The concentration and the specific radioactivity of 45Ca were 1 mM as CaCl2 and 20 MBq mmol−1, respectively. Circles pH 8.2; squares pH 7.7; diamonds pH 7.2
Effect of the acidification by HCl on 45Ca-uptake by the coccolithophore E. huxleyi under growth conditions. a, b Time course of 45Ca-uptake in the presence of various NaHCO3 concentrations at pH 8.2 and 7.7, respectively. The numbers beside of lines in a represent the final concentrations (mM) of NaHCO3 added to the medium: NA (not added, filled diamond), 1 (filled triangle), 2 (open diamond), 5 (open circle), 10 (filled circle). c Relationship between 45Ca-uptake activity during 24 h and the final concentrations of NaHCO3 added to the medium. The numbers beside of pH 8.2 line indicate the ratios of values at pH 8.2–7.7. d HCO3 − concentrations in the medium containing various concentrations (final) of NaHCO3 at pH 8.2 and 7.7. The equilibration of inorganic carbons was calculated by CO2 SYS
On the other hand, 45Ca-incorporation activity was stimulated by the addition of DIC (NaHCO3) regardless of concentration (Fig. 6a, b). Under such conditions, the 45Ca-uptake activity was largely stimulated and saturated with 5 mM NaHCO3 at pH 8.2, but not completely even with 10 mM at pH 7.7 while the extent of suppression by acidification was the largest at 1–2 mM DIC (Fig. 6c). These results indicate that the suppression of 45Ca-uptake by acidification with HCl can be recovered by the addition of NaHCO3, namely by the increase in bicarbonate concentration.
Effect of acidification on the production of coccolith polysaccharides by E. huxleyi
Acidification by CO2 enrichment stimulated the production of cellular contents of photosynthetic storage products such as neutral (NP) and acid (AP) polysaccharides, which are located in the cytoplasm and coccoliths, respectively, at pH 7.7 in comparison with pH 8.2 (Fig. 7a, b). On the other hand, the content of those polysaccharides was remarkably increased when acidification was attained by CO2 enrichment (Fig. 7d–f). The quantitative analytical data of NP and AP were also confirmed by SDS-PAGE images (Fig. 7c, g). The ratio of the amount of AP/NP was not affected by acidification with HCl (Fig. 7a, b), but NP production was more stimulated by acidification with CO2 enrichment (Fig. 7d–f).
Effect of the acidification by HCl (a, b) and the ocean acidification conditions by elevating pCO2 (c–e) on the production of polysaccharide and proteins by the coccolithophore E. huxleyi during 3 and 6 days under growth conditions. a, b At pH 8.2 and 7.7, respectively. d–f Under the bubbling of 406, 816 and 1,192 ppm CO2 in air of which pH attained were 8.0–8.3, 7.6–7.9 and 7.5–7.7, respectively, as indicated in the figure. Before experiments, cells had been grown at pH 8.2. White column acid polysaccharides (AP) determined by the carbazole-sulfuric acid method; vertical stripe column neutral polysaccharides (NP) calculated by the equation of [TP] − [AP]; hatched column total polysaccharides (TP) determined by the phenol–sulfuric acid method; black bar protein contents determined by the protein assay kit (Bio-Rad Laboratories AB). c, g Effect of acidification by HCl (c) and acidification by CO2 enrichment (g), respectively, on SDS-PAGE image for the production of neutral and acid polysaccharides by the coccolithophore E. huxleyi grown for 6 days. The amount of cell used for analysis was corresponded to 5 μg Chl. Total and acid polysaccharide bands were visualized by “Stains-all” and “Alcian blue,” respectively
Discussion
According to the IPCC scenario, oceanic pH is estimated to decrease 0.5 U, namely to pH 7.7, by 2100 (IPCC 2007). In addition to the effects of atmospheric CO2 elevation, acidification also can be seen at shallow coastal sites of volcanic CO2 vents. Along gradients of normal pH (8.1–8.2) to lowered pH (7.8–7.9, lowest 7.4–7.5), typical rocky shore communities with abundant calcareous organisms shifted to communities lacking scleractinian corals with significant reductions in sea urchin and coralline algal abundance (Hall-Spencer et al. 2008). If it happens in the surface ocean, coccolithophores will also be damaged and such damage of the primary producers in the ocean will change the composition of the global phytoplankton community and ecosystems. There are various views on the effect that ocean acidification has on calcification of the coccolithophore E. huxleyi. Algal growth was reported to be suppressed by acidification in coccolithophores, e.g., the decrease in the specific growth rate of coccolithophores at pH values below 8.0 (Swift and Taylor 1966). Iglesias-Rodriguez et al. (2008) reported that promotion of the calcification would happen by increase of the CO2. In contrast, Riebesell et al. (2000) described that the formation of the coccoliths will be inhibited by acidification.
In this study, we intended to compare the difference of acidification effect between acidification by acid supply and the bubbling of elevated concentrations of CO2 in order to observe how coccolithophores respond potentially to acidification. The experimental conditions set in this study were not exactly the same as those expected in ocean acidification since seawater contained buffers to induce change in alkalinity. Cell density was also very high, and the rate of bubbling was not strong enough to get complete equilibration of inorganic carbons. Therefore, while the data we obtained are not directly applicable to the determination of the effect of ocean acidification on coccolithophores in the ocean, the data are still useful to predict how coccolithophores will respond to acidification physiologically. For this purpose, we analyzed the whole effect of acidification on cell growth, photosynthetic O2 evolution, photosystem’s activity, Ca-uptake, the productivity of polysaccharides of AP and NP and coccolith production in the most abundant, bloom-forming coccolithophore, E. huxleyi.
When pH was simply decreased to 7.7 by acidification with HCl, the specific growth rate of E. huxleyi was diminished 31.2 % lower than that at pH 8.2 and they rapidly died within 1 day at pH 7.2 (Fig. 1a–d). In contrast, the acidification by CO2 enrichment by bubbling of 816 (lowest pH 7.6) and 1,192 ppm (lowest pH 7.5) slightly promoted algal growth (Fig. 1f–i). Those results indicate that E. huxleyi responds differently to acidification depending on whether it is accompanied by CO2 enrichment or not. The results also show that the diminution of algal growth by acidification with HCl can be overcome by an increase in CO2 supply.
Acidification shifts DIC equilibrium toward CO2, and therefore, the concentration of total DIC becomes low when pH is decreased in an open system (Fig. 1e). Interestingly, bicarbonate concentration calculated was almost similar at pH 8.2 and 7.7 at constant dissolved CO2 concentration under bubbling of air (Fig. 6d). The radiotracer experiment on 45Ca-uptake by E. huxleyi cells was performed to analyze the effect of acidification by HCl under bubbling of air with ca. 400 ppm. The results of the experiments clearly showed that 45Ca-uptake was strongly suppressed by acidification with HCl (Fig. 5). However, 45Ca-uptake was saturated with 5 mM DIC at pH 8.2, but not enough with 10 mM at pH 7.7 (Fig. 6c), indicating that high bicarbonate concentration is required for calcification. This result agrees with evidence showing that only bicarbonate, not CO2, is the substrate for intracellular calcification on E. huxleyi (Sekino and Shiraiwa 1994). Although the influence of acidification on calcification of E. huxleyi has been reported (Zondervan et al. 2001; Riebesell et al. 2000; Langer et al. 2006; Iglesias-Rodriguez et al. 2008), the mechanism how acidification changes physiological status of coccolithophores has not been studied in detail. Therefore, the present result gives important information to elucidate how acidification by acid and by CO2 enrichment will be different.
In unicellular green alga Mesotaenium caldariorum, the high rate of ATP-dependent Ca2+-uptake and direct Ca2+-transport/H+-antiport activities was found to be necessary for Ca2+ uptake (Berkelman and Lagarias 1990). Ca2+-permeable channels in the plasma membrane were suggested more likely to function for Ca2+ entry into calcifying coccolithophore cells (Brownlee and Taylor 2003). Ca2+ accumulation into the Golgi of eukaryotic cells occurs by H+/Ca2+ exchange driven by the inside acidic H+ electrochemical gradient across the Golgi membrane, which in turn is generated by V-type ATPase in eukaryotic cells (Harvey 1992). These previous reports show that acidification outside of membrane may disturb Ca2+ uptake through the Ca2+/H+ channel. The results support our conclusion that the suppression of Ca2+-uptake, calcification and coccolith production by E. huxleyi is due to the suppression of Ca2+-entry into cells by acidification of the medium (solid line in Fig. 8a). In addition, as the calcite saturation state is <1 in the low pH cultures, the coccoliths may also be dissolved even though coccoliths were produced and transported to the cell surface.
Schematic models showing the effect of acidification by HCl (a) and by bubbling of air with elevated CO2 (b) on CO2 equilibration, inorganic carbon utilization for photosynthesis, Ca-uptake and coccolith production by the coccolithophore E. huxleyi. The width of arrows represents the amount of compounds how much flow along the arrow. a Acidification by HCl changes in the equilibration between dissolved CO2 and bicarbonate toward CO2 production to decrease bicarbonate concentration. Dissolved CO2 concentration equilibrated with air bubbled was same among three pH conditions. The present study proved that the decrease in HCO3 − concentration suppressed coccolith production which is due to diminishing Ca2+-uptake by cells. Photosynthetic production of storage (NP) and coccolith polysaccharides (CP) was stimulated by acidification. b Acidification by CO2 enrichment increases dissolved CO2 concentration and bicarbonate production by increasing inorganic carbon substrates. The resulted increase in CO2 and bicarbonate which are substrate for photosynthetic CO2 fixation and intracellular calcification, respectively (Sekino and Shiraiwa 1994), stimulated both reactions. High concentration of bicarbonate also stimulated Ca-uptake. As a result, all those processes stimulated photosynthetic CO2 fixation and coccolith production
Acidification by CO2 enrichment promoted photosynthetic O2 evolution (Fig. 2), the morphological change in increase of cell volume, coccolith production (Fig. 4), Ca2+-uptake into cells (Fig. 6), and the production of acid (AP) and neutral polysaccharides (NP) (Fig. 7). On the other hand, acidification by both HCl and CO2 enrichment did not affect the activity of photosystem activities directly (Fig. 3).
The state of photosystem II determined by F v/F m and the electron transport activity of the whole photosystem (ϕPSII) of acidification were hardly changed by acidification during growth, irrespective of pH of the medium (Fig. 3a, b). In contrast, the F v/F m values decreased under ocean acidification conditions where air with elevated concentration of CO2 was bubbled (Fig. 3c, e). The reason for the difference is unclear yet. These data are different from a previous report in which damage of photosystem II (PSII), namely decrease in F v/F m, by acidification in the thylakoid membrane of the green algae Scenedesmus obliquus (Heinze and Dau 1996). The possible explanation on the phenomena is that excess CO2 concentration in the medium induces high CO2 input into the chloroplast stroma and results in rapid acidification by the conversion of CO2 to bicarbonate plus H+ by chloroplast carbonic anhydrases. Those reactions affect PSII directly and induced a decay of the F v/F m value.
Acid (AP), same as coccolith polysaccharide (CP), and neutral polysaccharides (NP) determined as parameters of coccolith and storage polysaccharides (Kayano and Shiraiwa 2009), respectively, are known as coccolith polysaccharides containing uronic acids and storage polysaccharides composed of β-1-3(1–6)-glucan, respectively (Mann 2001; Kayano and Shiraiwa 2009). The amount of AP and NP production was stimulated by acidification, but the AP/NP ratio was not affected (Fig. 7). These phenomena may be due to an increase of CO2 supply into the cells and consequently the stimulation of the production of acid polysaccharides. Such active AP production also may stimulate Ca2+-uptake by demand of Ca2+ to produce CaCO3 crystals for coccoliths. Both cell size and coccolith production were affected by acidification with CO2 concentration (Fig. 4). Cell enlargement was also observed when coccolith production was strongly stimulated at low temperature (Sorrosa et al. 2005). As swelling of the cells were observed when cell growth was greatly suppressed by nutrient-deficiency or cell damage (Satoh et al. 2009), cell enlargement by acidification with HCl to pH 7.2 might be due to cell damage. Satoh et al. (2009) and Kayano and Shiraiwa (2009) also reported that both coccolith and coccolith polysaccharide production were stimulated by phosphate deficiency from the medium, although the reason why cell size was enlarged by phosphate deprivation is still unclear.
Very recently, Bach et al. (2013) reported the results on analysis of impact of CO 2 and pH on the mechanism of photosynthesis and calcification in E. huxleyi and concluded that E. huxleyi is sensitive to low CO 2 and low bicarbonate as well as low pH beyond a limited tolerance range, but much less sensitive to elevated CO 2 and bicarbonate. These results nicely fit to our present results although the parameters determined experimentally in both studies were different. The experiments by Bach et al. (2013) were performed by following carbon chemistry exactly, and therefore, their results can be extrapolated to the real ocean to simulate how E. huxleyi will be affected by ocean acidification. The present study clearly proved the mechanism behind how and why calcification, namely coccoliths production, is stimulated at elevated CO2 conditions and inhibited under acidification. Therefore, the combination of both papers is useful to understand how and why ocean acidification by increasing atmospheric CO 2 will affect the physiology of the coccolithophore E. huxleyi.
In conclusion, the schematic model of the influence of acidification by acid (solid arrow) and by CO2 enrichment (open arrow) is shown in Fig. 8. The suppression of coccolith formation by acidification is shown to be due to the reduction of calcium uptake through the plasma membrane in E. huxleyi. On the other hand, photosynthetic machinery in the chloroplast was not affected by such acidification of the medium. This study proved that E. huxleyi cells have high potential of compensation to avoid damage of cells against acidification when acidification is caused by CO2 enrichment. This suggests that physiological activities of E. huxleyi cells will not be seriously damaged by ocean acidification at least up to 1,200 ppm CO2 in the atmosphere. However, as reported by Hoppe et al. (2011), there still is possibility that some ecophysiological difference and/or variation of E. huxleyi strains living in some specific habitats may induce some different response to ocean acidification.
References
Anthony KR, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification cause bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA 11:17442–17446
Bach LT, Mackinder LCM, Schulz KG, Wheeler G, Schroeder DC, Brownlee C, Riebesell U (2013) Dissecting the impact of CO2 and pH on the mechanism of photosynthesis and calcification in the coccolithophore Emiliania huxleyi. New Phytol 199:121–134
Berkelman T, Lagarias JC (1990) Calcium transport in the green alga Mesotaenium caldariorum. Plant Physiol 93:748–757
Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J (2007) Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol Lett 3:699–701
Bijma J, Hönisch B, Zeebe RE (2002) Impact of the ocean carbonate chemistry on living foraminiferal shell weight: “Comment on carbonate ion concentration in glacial-age deep waters of the Caribbean Sea” by W.S. Broecker and E. Clark. Geochem Geophys Geosyst 3:1064. doi:10.1029/2002GC000388
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334
Brownlee C, Taylor AR (2003) Calcification in coccolithophores: a cellular perspective. In: Thierstein H, Young J (eds) Coccolithophores: from molecular processes to global impact. Springer, Berlin, pp 31–50
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Danbara A, Shiraiwa Y (1999) The requirement of selenium for the growth of marine coccolithophorids, Emiliania huxleyi, Gephyrocapsa oceanica and Helladosphaera sp. (Prymnesiophyceae). Plant Cell Physiol 40:762–766
Demmig B, Bjorkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O, evolution in leaves of higher plants. Planta 171:171–184
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry V et al (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305:362–366
Gattuso JP, Frankignoulle M, Smith SV (1998) Measurement of community metabolism and significance in the coral reef CO2 source–sink debate. Proc Natl Acad Sci USA 96:13017–13022
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM et al (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Harvey WR (1992) Physiology of V-ATPases. J Exp Biol 172:1–17
Heinze I, Dau H (1996) The pH-dependence of the photosystem II fluorescence: cooperative transition to a quenching state. Ber Bunsenges Phys Chem 100:2008–2013
Hodge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrates. In: Whistler RL, Wolfrom MW (eds) Methods in carbohydrate chemistry, vol 1. Academic Press, New York, pp 380–394
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E et al (2007) Coral reefs under rapid climate change and ocean acidification. Science 321:51–52
Hoppe CJM, Langer G, Rost B (2011) Emiliania huxleyi shows identical response to elevated pCO2 in TA and DIC manipulations. J Exp Mar Biol Ecol 406:54–62
Iglesias-Rodriguez MD, Halloran PR, Rosalind EM, Rickaby REM, Hall IR, Colmenero-Hidalgo E et al (2008) Phytoplankton calcification in a High-CO2 world. Science 320:336–340
Intergovernmental Panel on Climate Change (IPCC) (2007) Summary for policy makers. In: Solomon S et al (eds) Climate change 2007: the physical sciences basis. Working group I contribution to the fourth assessment report of the IPCC. Cambridge University Press, Cambridge
Jeffrey SW (1972) Preparation and some properties of crystalline chlorophyll c 1 and c 2 from marine algae. Biochim Biophys Acta 279:15–33
Kayano K, Shiraiwa Y (2009) Physiological regulation of coccolith polysaccharide production by phosphate availability in the coccolithophorid Emiliania huxleyi. Plant Cell Physiol 50:1522–1531
Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Kuffner IB, Andersson AJ, Jokiel PL, Rodgers K, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Langer G, Geisen M, Baumann KH, Klas J, Riebesell U, Thoms S et al (2006) Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem Geophys Geosyst 7. doi:10.1029/2005GC001227
Leuker TJ, Dickson AG, Keeling CD (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K 1 and K 2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem 70:105–119
Liu H, Probert I, Uitz J, Claustre H, Aris-Brosou S, Frada M et al (2009) Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. Proc Natl Acad Sci USA 106:12803–12808
Mann S (2001) Biomineralization: principles and concepts in bioinorganic materials chemistry. Oxford University Press, Oxford
Miles H, Widdicombe S, Spicer JI, Hall-Spencer J (2007) Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar Pollut Bull 54:89–96
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Read BA, Kegel J, Klute MJ, Kuo A, Lefebvre SC, Maumus F et al (2013) Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 499:209–213
Richier S, Fiorini S, Kerros ME, Peter von Dassow PV, Gattuso JP (2010) Response of the calcifying coccolithophore Emiliania huxleyi to low pH/high pCO2: from physiology to molecular level. Mar Biol 158:551–560
Riebesell U, Zondervan RostB, Tortell PD, Zeebe RE, Morel FMM (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407:364–367
Ross PM, Parker L, O’Conner WA, Bailey EA (2011) The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 3:1005–1030
Satoh M, Iwamoto K, Suzuki I, Shiraiwa Y (2009) Cold stress stimulates intracellular calcification by the coccolithophore, Emiliania huxleyi (Haptophyceae) under phosphate-deficient conditions. Mar Biotechnol 11:327–333
Sekino K, Shiraiwa Y (1994) Accumulation and utilization of dissolved inorganic carbon by a marine unicellular coccolithophorid, Emiliania huxleyi. Plant Cell Physiol 35:353–361
Sorrosa JM, Satoh M, Shiraiwa Y (2005) Low temperature stimulates cell enlargement and intracellular calcification of coccolithophorids. Mar Biotechnol 7:128–133
Swift E, Taylor W (1966) The effect of pH on the division rate of the coccolithophorid Cricosphaera elongata. J Phycol 2:121–125
Veron JE, Hoegh-Guldberg O, Lenton TM, Lough JM, Obura DO, Pearce-Kelly P et al (2009) The coral reef crisis: the critical importance of <350 ppm CO2. Mar Pollut Bull 58:1428–1436
Zeebe RE, Zachos JC, Calderia K, Tyrrell T (2008) Carbon emissions and acidification. Science 321:51–52
Zondervan I, Zeebe RE, Rost B, Riebesell U (2001) Decreasing marine biogenic calcification: a negative feedback on rising atmospheric pCO2. Glob Biogeochem Cycles 15:507–551
Acknowledgments
We thank that Dr. T. Midorikawa of the Meteorological Research Institute, Japan, for providing data on the equilibration of DIC species in the medium at various pHs. We also appreciate very much for valuable suggestion and discussion to Dr. J. Toney of the University of Glasgow and anonymous reviewers. This study was supported in part by the Global Environment Research Fund from the Japanese Ministry of Environment to YS (FY2008-2010, F-083), the grant-in-aid of the Basic Research Area (S) by JSPS and MEXT to YS (FY2010-14) and the CREST, JST to YS (FY2011-15).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Fukuda, Sy., Suzuki, Y. & Shiraiwa, Y. Difference in physiological responses of growth, photosynthesis and calcification of the coccolithophore Emiliania huxleyi to acidification by acid and CO2 enrichment. Photosynth Res 121, 299–309 (2014). https://doi.org/10.1007/s11120-014-9976-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-9976-9