Abstract
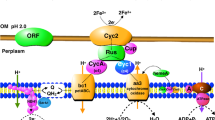
Phototrophs of the family Heliobacteriaceae contain the simplest known Type I reaction center (RC), consisting of a homodimeric (PshA)2 core devoid of bound cytochromes and antenna proteins. Unlike plant and cyanobacterial Photosystem I in which the FA/FB protein, PsaC, is tightly bound to P700–FX cores, the RCs of Heliobacterium modesticaldum contain two FA/FB proteins, PshBI and PshBII, which are loosely bound to P800–FX cores. These two 2[4Fe–4S] ferredoxins have been proposed to function as mobile redox proteins, reducing downstream metabolic partners much in the same manner as does [2Fe–2S] ferredoxin or flavodoxin (Fld) in PS I. Using P800–FX cores devoid of PshBI and PshBII, we show that iron–sulfur cluster FX directly reduces Fld without the involvement of FA or FB (Fld is used as a proxy for soluble redox proteins even though a gene encoding Fld is not identified in the H. modesticaldum genome). The reduction of Fld is suppressed by the addition of PshBI or PshBII, an effect explained by competition for the electron on FX. In contrast, P700–FX cores require the presence of the PsaC, and hence, the FA/FB clusters for Fld (or ferredoxin) reduction. Thus, in H. modesticaldum, the interpolypeptide FX cluster serves as the terminal bound electron acceptor. This finding implies that the homodimeric (PshA)2 cores should be capable of donating electrons to a wide variety of yet-to-be characterized soluble redox partners.



Similar content being viewed by others
References
Amesz J (1995) The heliobacteria, a new group of photosynthetic bacteria. J Photochem Photobiol B 30(2–3):89–96
Azai C, Tsukatani Y, Itoh S, Oh-oka H (2010) C-type cytochromes in the photosynthetic electron transfer pathways in green sulfur bacteria and heliobacteria. Photosynth Res 104:189–199
Brettel K, Leibl W, Liebl U (1998) Electron transfer in the heliobacterial reaction center: evidence against a quinone-type electron acceptor functioning analogous to A1 in photosystem I. Biochim Biophys Acta 1363(3):175–181
Brockmann H, Lipinski A (1983) Bacteriochlorophyll-g a new bacteriochlorophyll from Heliobacterium chlorum. Arch Microbiol 136(1):17–19
Fillat MF, Sandmann G, Gomez-Moreno C (1988) Flavodoxin from the nitrogen-fixing cyanobacterium Anabaena PCC 7119. Arch Microbiol 150:160–164
Gest H, Favinger JL (1983) Heliobacterium chlorum, an anoxygenic brownish-green photosynthetic bacterium containing a new form of bacteriochlorophyll. Arch Microbiol 136(1):11–16
Heinnickel M, Golbeck JH (2007) Heliobacterial photosynthesis. Photosynth Res 92(1):35–53
Heinnickel M, Shen G, Agalarov R, Golbeck JH (2005) Resolution and reconstitution of a bound Fe–S protein from the photosynthetic reaction center of Heliobacterium modesticaldum. Biochemistry 44(29):9950–9960
Heinnickel M, Agalarov R, Svensen N, Krebs C, Golbeck JH (2006) Identification of FX in the heliobacterial reaction center as a [4Fe–4S] cluster with an S = 3/2 ground spin state. Biochemistry 45(21):6756–6764
Heinnickel M, Shen G, Golbeck JH (2007) Identification and characterization of PshB, the dicluster ferredoxin that harbors the terminal electron acceptors FA and FB in Heliobacterium modesticaldum. Biochemistry 46(9):2530–2536
Jagannathan B, Golbeck JH (2008) Unifying principles in homodimeric type I photosynthetic reaction centers: properties of PscB and the FA, FB and FX iron–sulfur clusters in green sulfur bacteria. Biochim Biophys Acta 1777(12):1535–1544
Jagannathan B, Shen G, Golbeck J (2011) Evolution of Photosystem I. In: Burnap R, Vermaas W (eds) Functional genomics and evolution of photosynthetic systems. Springer, Dordrecht
Kleinherenbrink FA, Amesz J (1993) Stoichiometries and rates of electron transfer and charge recombination in Heliobacterium chlorum. Biochem Biophys Acta 1143:77–83
Kleinherenbrink FA, Ikegami I, Hirashi A, Otte SCM, Amesz J (1993) Electron transfer in menaquinone-depleted membranes of Heliobacterium chlorum. Biochem Biophys Acta 1142:69–73
Kleinherenbrink FA, Chiou HC, LoBrutto R, Blankenship RE (1994) Spectroscopic evidence for the presence of an iron–sulfur center similar to Fx of Photosystem I in Heliobacillus mobilis. Photosynth Res 41(1):115–123
Kobayashi M, van de Meent EJ, Erkelens C, Amesz J, Ikegami I, Watanabe T (1991) Bacteriochlorophyll g epimer as a possible reaction center component of heliobacteria. Biochem Biophys Acta 1057:89–96
Liebl U, Mockensturmwilson M, Trost JT, Brune DC, Blankenship RE, Vermaas W (1993) Single core polypeptide in the reaction center of the photosynthetic bacterium Heliobacillus mobilis—structural implications and relations to other photosystems. Proc Natl Acad Sci USA 90(15):7124–7128
Lin S, Chiou HC, Kleinherenbrink FA, Blankenship RE (1994) Time-resolved spectroscopy of energy and electron transfer processes in the photosynthetic bacterium Heliobacillus mobilis. Biophys J 66(2 Pt 1):437–445
Medina M, Hervas M, Navarro JA, De la Rosa MA, Gomez-Moreno C, Tollin G (1992) A laser flash absorption spectroscopy study of Anabaena sp. PCC 7119 flavodoxin photoreduction by photosystem I particles from spinach. FEBS Lett 313(3):239–242
Meimberg K, Muhlenhoff U (1999) Laser-flash absorption spectroscopy study of the competition between ferredoxin and flavodoxin photoreduction by Photosystem I in Synechococcus sp. PCC 7002: evidence for a strong preference for ferredoxin. Photosynth Res 61:253–2677
Meimberg K, Fischer N, Rochaix JD, Muhlenhoff U (1999) Lys35 of PsaC is required for the efficient photoreduction of flavodoxin by photosystem I from Chlamydomonas reinhardtii. Eur J Biochem 263(1):137–144
Miyamoto R, Iwaki M, Mino H, Harada J, Itoh S, Oh-Oka H (2006) ESR signal of the iron–sulfur center FX and its function in the homodimeric reaction center of Heliobacterium modesticaldum. Biochemistry 45(20):6306–6316
Miyamoto R, Mino H, Kondo T, Itoh S, Oh-Oka H (2008) An electron spin-polarized signal of the P800+ A1(Q)− state in the homodimeric reaction center core complex of Heliobacterium modesticaldum. Biochemistry 47(15):4386–4393
Muh F, Glockner C, Hellmich J, Zouni A (2012) Light-induced quinone reduction in photosystem II. Biochim Biophys Acta 1817(1):44–65
Muhiuddin IP, Rigby SE, Evans MC, Amesz J, Heathcote P (1999) ENDOR and special TRIPLE resonance spectroscopy of photoaccumulated semiquinone electron acceptors in the reaction centers of green sulfur bacteria and heliobacteria. Biochemistry 38(22):7159–7167
Naver H, Scott MP, Golbeck JH, Olsen CE, Scheller HV (1998) The eight-amino acid internal loop of PSI-C mediates association of low molecular mass iron–sulfur proteins with the P700–FX core in photosystem I. J Biol Chem 273(30):18778–18783
Neerken S, Amesz J (2001) The antenna reaction center complex of heliobacteria: composition, energy conversion and electron transfer. Biochim Biophys Acta 1507(1–3):278–290
Nitschke W, Setif P, Liebl U, Feiler U, Rutherford AW (1990) Reaction center photochemistry of Heliobacterium chlorum. Biochemistry 29(50):11079–11088
Nuijs AM, van Dorssen RJ, Duysens LNM, Amesz J (1985) Excited states and primary photochemical reactions in the photosynthetic bacterium Heliobacerium chlorum. Proc Natl Acad Sci USA 82:6865–6868
Oh-oka H (2007) Type 1 reaction center of photosynthetic heliobacteria. Photochem Photobiol 83(1):177–186
Romberger SP, Golbeck JH (2010) The bound iron–sulfur clusters of type-I homodimeric reaction centers. Photosynth Res 104(2–3):333–346
Romberger SP, Castro C, Sun Y, Golbeck JH (2010) Identification and characterization of PshBII, a second FA/FB-containing polypeptide in the photosynthetic reaction center of Heliobacterium modesticaldum. Photosynth Res 104(2–3):293–303
Sattley WM, Madigan MT, Swingley WD, Cheung PC, Clocksin KM, Conrad AL, Dejesa LC, Honchak BM, Jung DO, Karbach LE, Kurdoglu A, Lahiri S, Mastrian SD, Page LE, Taylor HL, Wang ZT, Raymond J, Chen M, Blankenship RE, Touchman JW (2008) The genome of Heliobacterium modesticaldum, a phototrophic representative of the Firmicutes containing the simplest photosynthetic apparatus. J Bacteriol 190(13):4687–4696
Stevenson AK, Kimble LK, Woese CR, Madigan MT (1997) Characterization of new phototrophic heliobacteria and their habitats. Photosynth Res 53:1–12
Trost JT, Blankenship RE (1989) Isolation of a photoactive photosynthetic reaction center-core antenna complex from Heliobacillus mobilis. Biochemistry 28(26):9898–9904
van de Meent EJ, Kobayashi M, Erkelens C, van Veelen PA, Amesz J, Watanabe T (1991) Identification of 8-hydroxychlorophyll a as a function reaction center pigment in heliobacteria. Biochem Biophys Acta 1058:356–362
van der Est A, Hager-Braun C, Leibl W, Hauska G, Stehlik D (1998) Transient electron paramagnetic resonance spectroscopy on green-sulfur bacteria and heliobacteria at two microwave frequencies. Biochim Biophys Acta 1409(2):87–98
Vermaas WFJ (1994) Evolution of heliobacteria—implications for photosynthetic reaction-center complexes. Photosynth Res 41(1):285–294
Woese CR, Debrunnervossbrinck BA, Oyaizu H, Stackebrandt E, Ludwig W (1985) Gram-positive bacteria—possible photosynthetic ancestry. Science 229(4715):762–765
Xu Q, Jung YS, Chitnis VP, Guikema JA, Golbeck JH, Chitnis PR (1994) Mutational analysis of photosystem I polypeptides in Synechocystis sp. PCC 6803. Subunit requirements for reduction of NADP+ mediated by ferredoxin and flavodoxin. J Biol Chem 269(34):21512–21518
Zhao J, Li R, Bryant DA (1998) Measurement of photosystem I activity with photoreduction of recombinant flavodoxin. Anal Biochem 264(2):263–270
Acknowledgment
This study is supported by a grant from the U.S. Department of Energy Chemical Sciences, Geosciences, & Biosciences Division (DE-FG02-98ER20314).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romberger, S.P., Golbeck, J.H. The FX iron–sulfur cluster serves as the terminal bound electron acceptor in heliobacterial reaction centers. Photosynth Res 111, 285–290 (2012). https://doi.org/10.1007/s11120-012-9723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9723-z