Abstract
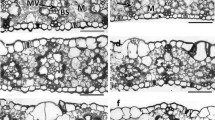
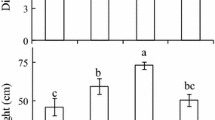
The potential role of foliar carbon export features in the acclimation of photosynthetic capacity to differences and changes in light environment was evaluated. These features included apoplastic vs. symplastic phloem loading, density of loading veins, plasmodesmatal frequency in intermediary cells, and the ratio of loading cells to sieve elements. In initial studies, three apoplastic loaders (spinach, pea, Arabidopsis thaliana) exhibited a completely flexible photosynthetic response to changing light conditions, while two symplastic loaders (pumpkin, Verbascum phoeniceum), although able to adjust to different long-term growth conditions, were more limited in their response when transferred from low (LL) to high (HL) light. This suggested that constraints imposed by the completely physical pathway of sugar export might act as a bottleneck in the export of carbon from LL-acclimated leaves of symplastic loaders. While both symplastic loaders exhibited variable loading vein densities (low in LL and high in HL), none of the three apoplastic loaders initially characterized exhibited such differences. However, an additional apoplastic species (tomato) exhibited similar differences in vein density during continuous growth in different light environments. Furthermore, in contrast to the other apoplastic loaders, photosynthetic acclimation in tomato was not complete following a transfer from LL to HL. This suggests that loading vein density and loading cells per sieve element, and thus apparent loading surface capacity, play a major role in the potential for photosynthetic acclimation to changes in light environment. Photosynthetic acclimation and vein density acclimation were also characterized in the slow-growing, sclerophytic evergreen Monstera deliciosa. This evergreen possessed a lower vein density during growth in LL compared to HL and exhibited a more severely limited potential for photosynthetic acclimation to increases in light environment than the rapidly-growing, mesophytic annuals.









Similar content being viewed by others
Abbreviations
- A:
-
Antheraxanthin
- CC:
-
Companion cell
- HL:
-
High light
- LL:
-
Low light
- PC:
-
Phloem parenchyma cell
- PFD:
-
Photon flux density
- SE:
-
Sieve element
- TC:
-
Transfer cell
- V:
-
Violaxanthin
- Z:
-
Zeaxanthin
References
Adams WW III, Demmig-Adams B (1992) Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 186:390–398
Adams WW III, Demmig-Adams B (2004) Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 583–604
Adams WW III, Demmig-Adams B, Winter K, Schreiber U (1990) The ratio of variable to maximum chlorophyll fluorescence from photosystem II, measured in leaves at ambient temperature and at 77K, as an indicator of the photon yield of photosynthesis. Planta 180:166–174
Adams WW III, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V (2002) Photosynthesis and photoprotection in overwintering plants. Plant Biol 4:545–557
Adams WW III, Amiard VSE, Mueh KE, Turgeon R, Demmig-Adams B (2005) Phloem loading type and photosynthetic acclimation to light. In: van der Est A, Bruce D (eds) Photosynthesis: fundamental aspects to global perspectives. Allen Press, Lawrence, pp 814–816
Adams WW III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2006) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol 21. Springer, Dordrecht, pp 49–64
Amiard V, Mueh KE, Demmig-Adams B, Ebbert V, Turgeon R, Adams WW III (2005) Anatomical and photosynthetic acclimation to light environment in species with differing mechanisms of phloem loading. Proc Natl Acad Sci USA 102:12968–12973
Amiard V, Demmig-Adams B, Mueh KE, Turgeon R, Combs AF, Adams WW III (2007) Role of light and jasmonic acid signaling in regulating foliar phloem cell wall ingrowth development. New Phytol 173
Beebe DU, Turgeon R (1992) Localization of glactinol, raffinose, and stachyose synthesis in Cucurbita pepo leaves. Planta 188:354–361
Buchi R, Bachmann M, Keller F (1998) Carbohydrate metabolism in source leaves of sweet basil (Ocimum basilicum L.), a starch storing and stachyose translocating labiate. J Plant Physiol 153:308–315
Delieu T, Walker DA (1981) Polarographic measurement of photosynthetic oxygen evolution by leaf disks. New Phytol 89:165–178
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal dissipation. New Phytol 172:11–21
Demmig-Adams B, Winter K, Winkelmann E, Krüger A, Czygan F-C (1989) Photosynthetic characteristics and the ratios of chlorophyll, b-carotene, and the components of the xanthophyll cycle upon a sudden increase in growth light regime in several plant species. Bot Acta 102:319–325
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the allocation of absorbed light to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Demmig-Adams B, Moeller DL, Logan BA, Adams WW III (1998) Positive correlation between levels of retained zeaxanthin + antheraxanthin and degree of photoinhibition in shade leaves of Schefflera arboricola. Planta 205:367–374
Demmig-Adams B, Ebbert V, Mellman DL, Mueh KE, Schaffer L, Funk C, Zarter CR, Adamska I, Jansson S, Adams WW III (2006a) Modulation of PsbS and flexible versus sustained energy dissipation by light environment in different species. Physiol Plant 127:670–680
Demmig-Adams B, Ebbert V, Zarter CR, Adams WW III (2006b) Characteristics and species-dependent employment of flexible versus sustained thermal dissipation and photoinhibition. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol 21. Springer, Dordrecht, pp 39–48
Dijkwel PP, Kock PAM, Bezemer R, Weisbeek PJ, Smeekens SCM (1996) Sucrose represses the developmentally controlled transient activation of the plastocyanin gene in Arabidopsis thaliana seedlings. Plant Physiol 110:455–463
Ebbert V, Demmig-Adams B, Adams WW III, Mueh KE, Staehelin LA (2001) Association between persistent forms of zeaxanthin-dependent energy dissipation and thylakoid protein phosphorylation. Photosynth Res 67:63–78
Essau K (1977) Anatomy of seed plants. John Wiley and Sons, New York
Gamalei Y (1989) Structure and function of leaf minor veins in trees and herbs. Trees – Struct Funct 3:96–110
Gamalei Y (1991) Phloem loading and its development related to plant evolution from trees to herbs. Trees – Struct Funct 5:50–64
Gilmore AM, Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J Chromatogr 543:137–145
Goggin FL, Medville R, Turgeon R (2001) Phloem loading in the tulip tree. Mechanisms and evolutionary implications. Plant Physiol 125:891–899
Haritatos E, Turgeon R (1995) Symplastic phloem loading by polymer trapping. In: Pontis HG, Salerno GL, Echeverria EJ (eds) Sucrose metabolism, biochemistry, physiology and molecular biology. American Society of Plant Physiology, Rockville, pp 216–224
Haritatos E, Keller F, Turgeon R (1996) Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L. leaves: Implications for phloem loading. Planta 198:614–622
Haritatos E, Medville R, Turgeon R (2000) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211:105–111
Holthaus U, Schmitz K (1991) Distribution and immunolocalization of stachyose synthase in Cucumis melo L. Planta 185:479–486
Jones PG, Lloyd JC, Raines CA (1996) Glucose feeding of intact wheat plants represses the expression of a number of Calvin cycle genes. Plant Cell Environ 19:231–236
Kilb B, Wietoska H, Godde D (1996) Changes in the expression of photosynthetic genes precede loss of photosynthetic activities and chlorophyll when glucose is supplied to mature spinach leaves. Plant Sci 115:225–235
Kitajima M, Butler WL (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376:105–115
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540
Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the ‘sink-regulation’ of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195:313–323
Krapp A, Hofmann B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates – a mechanism for the sink regulation of photosynthesis. Plant J 3:817–828
Kühn C (2003) A comparison of the sucrose transporter systems of different plant species. Plant Biol 5:215–232
Lalonde S, Tegeder M, Throne-Holse M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26:37–56
Layne DR, Flore JA (1995) End-product inhibition of photosynthesis in Prunus cerasus L. in response to whole-plant source-sink manipulation. J Am Soc Hort Sci 120:583–599
Logan BA, Demmig-Adams B, Rosenstiel TN, Adams WW III (1999) Effect of nitrogen limitation on foliar antioxidants in relationship to other metabolic characteristics. Planta 209:213–220
Lohaus G, Winter H, Riens B, Heldt HW (1995) Further studies of the phloem loading process in leaves of barley and spinach – The comparison of metabolite concentrations in the apoplastic compartment with those in the cytosolic compartment and in the sieve tubes. Bot Acta 108:270–275
Makino A, Mae T (1999) Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol 40:999–1006
Moore DB, Palmquist DE, Seemann JR (1997) Influence of plant growth at high CO2 concentrations on leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase and intracellular distribution of soluble carbohydrates in tobacco, snapdragon, and parsley. Plant Physiol 115:241–246
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2003) Transfer cells: cells specialized for a special purpose. Annu Rev Plant Biol 54:431–454
Paul MJ, Driscoll SP (1997) Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ 20:110–116
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 129:S185–S205
Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51:49–81
Sondergaard TE, Schulz A, Palmgren MG (2004) Energization of transport processes in plants. Roles of the plasma membrane H+-ATPases. Plant Physiol 136:2475–2482
Stitt M, von Schaewen A, Willmitzer L (1991) Sink regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in their cell wall involves a decrease of the Calvin cycle enzymes and an increase of glycolytic enzymes. Planta 183:40–50
Turgeon R, Gowan E (1990) Phloem loading in Coleus blumei in the absence of carrier-mediated uptake of export sugar from the apoplast. Plant Physiol 94:1244–1249
Turgeon R, Beebe DU, Gowan E (1993) The intermediary cell: minor vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191:446–456
van Bel AJE (1993) Strategies of phloem loading. Annu Rev Plant Physiol Plant Mol Biol 44:253–281
van Oosten J-J, Besford RT (1996) Acclimation of photosynthesis to elevated CO2 through feedback regulation of gene expression: climate of opinion. Photosynth Res 48:353–365
Volk GM, Turgeon R, Beebe DU (1996) Secondary plasmodesmata formation in the minor-vein phloem of Cucumis melo L. and Cucurbita pepo L. Planta 199:425–432
Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12:1345–1355
Wimmers LE, Turgeon R (1991) Transfer cells and solute uptake in minor veins of Pisum sativum leaves. Planta 186:2–12
Acknowledgments
The financial support of the National Science Foundation (Awards IBN-0235351 to W.W.A. and B.D.-A. and IBN-0235709 to R.T.) and the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture (Agreement No. 00-35100-9564) is gratefully acknowledged. We thank Dr. Tom Giddings for his support and guidance with electron microscopy, and Drs. Todd N. Rosenstiel and Brandon Moore for the carbohydrate analyses of M. deliciosa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adams, W.W., Watson, A.M., Mueh, K.E. et al. Photosynthetic acclimation in the context of structural constraints to carbon export from leaves. Photosynth Res 94, 455–466 (2007). https://doi.org/10.1007/s11120-006-9123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-006-9123-3