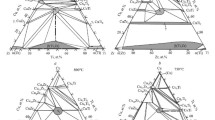
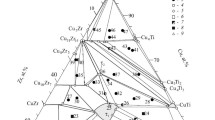
The Cu–Ti–Zr system and associated multicomponent systems are of practical interest as their alloys show high bulk glass forming ability. The Cu–Ti–Zr system is divided into two independent subsystems (Ti–CuTi2–CuZr2–Zr and Cu–CuTi2–CuZr) by the quasibinary vertical CuTi2–CuZr2 section. In this paper, phase equilibria in the Ti–CuTi2–CuZr2–Zr subsystem were experimentally studied. The structure of the as-cast binary and ternary alloys and the temperature of phase transformations in the samples that were cast and annealed at 750°C were studied by physicochemical analysis methods. The results were used to construct the liquidus and solidus surfaces, phase diagram, and vertical sections with 10, 20, and 30 at.% Cu, confirm the congruent formation of binary CuTi2 and CuZr2 compounds at 1012 and 1000°C, and find the composition and temperature of invariant eutectic reactions with their participation. The liquidus surface consists of two primary crystallization surfaces of infinite series of (βTi, βZr) (β) and Cu (Ti, Zr)2 (γ) phases, which intersect along the univariant eutectic curve. The liquidus temperatures decrease from the boundary binary systems to the ternary one, reaching the minimum at 845 °C. The solidus surface is characterized by the coexistence of the β and γ phases (β + γ range) over the entire composition range. The copper solubility is from 5 to 8 at.% in the β phase and up to 2 at.% in the γ phase. This two-phase region is formed through the eutectic L ⇄ (βTi, βZr) + Cu (Ti, Zr)2 reaction. There is also a minimum at 845°C on the solidus surface. The compositions of the two solid and one liquid phases coexisting at the minimum temperature are found on a single tie-line, along which the three-phase equilibrium is invariant. The compositions of the phases in this equilibrium are as follows: Lmin—Cu30Ti37Zr33, βmin—Cu10.5Ti62Zr28.5, and γmin—Cu32Ti35Zr33 (at.%). According to differential thermal analysis, the minimum temperature of the eutectoid (βTi, βZr) ⇄ (αTi, αZr) + Cu(Ti,Zr)2 transformation is 570°C.







Similar content being viewed by others
Notes
Composition of the alloys and phases is given in at.%
References
A. Inoue, W. Zhang, T. Zhang, and K. Kurosaka, “Cu-based bulk glassy alloys with high tensile strength of over 2000 MPa,” J. Non-Cryst. Solids, No. 304, 200–209 (2002).
P.-Y. Lee, Y.-M. Cheng, J.-Y. Chen, and C.-J. Hu, “Formation and corrosion behavior of mechanically-alloyed Cu–Zr–Ti bulk metallic glasses,” Metals, No. 7, 148 (2017).
X. Gao, X. Lin, Q. Yan, Z. Wang, X. Yu, Y. Zhou, Y. Hu, and W. Huang, “Effect of Cu content on microstructure and mechanical properties of in-situ phases reinforced Ti/Zr-based bulk metallic glass matrix composite by selective laser melting (SLM),” J. Mater. Sci. Technol., No. 67, 174–185 (2021).
G.N. Hermana, H.-M. Hsiao, P.-C. Kuo, P.K. Liaw, Y.-C. Li, S. Iikubo, and Y.-W. Yen, “Phase equilibria of the Cu–Zr–Ti ternary system at 703°C and the thermodynamic assessment and metallic glass region prediction of the Cu–Zr–Ti ternary system,” J. Non-Cryst. Solids, 551, 20387 (2021).
E.A. Ence and H.A. Margolin, “A study of the Ti–Cu–Zr system and the structure of Ti2Cu,” Trans. Metall. Soc. AIME, 221, 320–322 (1961).
M.Yu. Teslyuk, Intermetallic Compounds with the Laves Phase Structure [in Russian], Nauka, Moscow (1969), p. 138.
C.G. Woychik and T.B. Massalski, “Phase diagram relationships in the system Copper–Titanium–Zirconium,” Z. Metallkd., 79, No. 3, 149-153 (1988).
V.N. Chebotnikov and V.V. Molokanov, “Structure and properties of amorphous and crystalline alloys in the Ti2Cu–Zr2Cu section of the Ti–Zr–Cu system,” Izv. Akad. Nauk SSSR. Neorg. Mater., 26, No. 5, 960-964 (1990).
Yu.K. Kovneristy and A.G. Pashkovska, “Bulk glass formation of alloys in the intermetallic-containing Ti–Cu–Zr system,” Amorf. (Stekloobraz.) Met. Mater., Inst. Metall. RAN, Moscow (1992), pp. 153-157.
R. Arroyave, T.W. Eagar, and L. Kaufman, “Thermodynamic assessment of the Cu–Ti–Zr system,” J. Alloys Compd., 351, No. 1-2, 158-170 (2003).
T. Velikanova and M. Turchanin, “Copper–Titanium–Zirconium,” in: Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology (New Series), W. Martienssen (ed.), Group IV: Physical Chemistry, Ternary Alloy Systems, Phase Diagrams, Crystallographic and Thermodynamic Data, Springer-Verlag, Germany, Berlin, Heidelberg (2006), Vol. 11C3, pp. 436–464.
A.M. Storchak–Fedyuk, V.M. Petyukh, and L.V. Artyukh, “Study of cast alloys in the Cu–Ti–Zr system,” Sovr. Probl. Materialoved. Ser. Fiz-Khim. Osn. Tekhnol. Poroshl. Mater., 22–26 (2007).
P.G. Qin, H. Wang, H.S. Zhang, H.S. Liu, and Z.P. Jin, “The isothermal section of the Cu–Ti–Zr system at 1023 K measured with diffusion-triple approach,” Mater. Sci. Eng. A, 476, 83-88 (2008).
A.M. Storchak–Fedyuk, L.V. Artyukh, L.A. Duma, P.G. Agraval, M.A. Turchanin, and T.Ya. Velikanova, “Phase equilibria in the Cu–Ti–Zr system at 750°C. I. The isothermal section with copper content from 0 to 50 at.%,” Powder Metall. Met. Ceram., 56, No. 1–2, 78–87 (2017).
A.M. Storchak–Fedyuk, L.V. Artyukh, A.V. Grytsiv, P.G. Agraval, M.A. Turchanin, and T.Ya. Velikanova, “Phase equilibria in the Cu–Ti–Zr system at 750°C. II. The isothermal section with copper content from 50 to 100 at.%,” Powder Metall. Met. Ceram., 56, No. 3–4, 220–230 (2017).
W.-R. Chiang, K.-C. Hsieh, Y.A. Chang, G. Fan, D. Qiao, F. Jiang, and P.K. Liaw, “Phase equilibrium in the Cu–Ti–Zr system at 800°C,” Mater. Trans., 48, No. 7, 1631-1634 (2007).
U.E. Klotz, C. Liu, P.J. Uggowitzer, and J.F. Leoffler, “Experimental investigation of the Cu-Ti-Zr system at 800°C,” Intermetallics, 15, 1666-1671 (2007).
M.A. Turchanin, T.Ya. Velikanova, P.G. Agraval, A.R. Abdulov, and L.A. Dreval, “Thermodynamic assessment of the Cu-Ti-Zr system: III. Cu-Ti-Zr system,” Powder Metall. Met. Ceram., 47, No. 9-10, 586-606 (2008).
I. Ansara and V. Ivanchenko, “Cu–Ti (copper-titanium),” in: G. Effenberg (ed.), MSIT Binary Evaluation Program, Materials, MSI, MSIT Workplace.
T.V. Massalski, P.R. Subramanian, H. Okomoto, and L. Kasprzak (eds.), Binary Alloy Phase Diagrams: Handbook, 3 vols., 2nd ed., ASM Int. Materials Park, Ohio, USA (1990), p. 3589.
M.A. Turchanin, P.G. Agraval, and A.R. Abdulov, “Thermodynamic assessment of the Cu–Ti–Zr system: II. Cu–Zr and Ti–Zr systems,” Powder Metall. Met. Ceram., 47, No. 7–8, 428–446 (2008).
D. Arias and J.P. Abriata, “Cu–Zr (copper–zirconium),” in: P.R. Subramanian, D.J. Chakrabarti, and D.E. Laughlin (eds.), Phase Diagrams of Binary Copper Alloys, ASM Materials Park, Ohio, USA (1994), pp. 497–502.
E. Kneller, Y. Khan, and U. Corres, “The alloy system Copper-Zirconium. Part I. Phase diagrams and structural relations,” Z. Metallkd., 77, 43–48 (1986).
P. Villars and L.D. Calvert, Pearson’s Handbook of Crystallographic Data for Intermetallic Phases, 2nd ed., 4 vols., ASM Int. Materials Park, Ohio, USA (1991).
S.P. Alisova, N.V. Lutska, A.N. Kobylkin, and P.B. Budgerg, “The TiFe-Ti2Cu section in the Ti-Fe-Cu system. Conditions for the formation of Ti2Cu,” Metally, No. 5, 171–173 (1994).
A.R. Abdulov, M.A. Turchanin, P.G. Agraval, and A.A. Solorev, “Mixing enthalpy of liquid alloys in the Cu–Ti–Zr system,” Metally, No. 1, 28–34 (2007).
Yu.A. Kocherzhynski, E.A. Shishkin, and V.I. Vasilenko, “DTA apparatus with a thermocouple sensor to 2200°C,” in: N.V. Ageev and O.S. Ivanov (eds.), Phase Diagrams of Metallic Systems [in Russian], Nauka, Moscow (1971), pp. 245–249.
K.C.H. Kumar, I. Ansara, P. Wollants, and L. Delaey, “Thermodynamic optimization of the Cu–Ti system,” Z. Metallkd., 87, No. 8, 666–672 (1996).
V.N. Eremenko, Yu.I. Buyanov, and S.B. Prima, “Phase diagram of the system titanium–copper,” Powder Metall. Met. Ceram., 5, No. 6, 494–502 (1966).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 61, Nos. 5–6 (545), pp. 99–113, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Storchak, A., Velikanova, T.Y., Petyukh, V. et al. Phase Equilibria in the Ti–CuTi2–CuZr2–Zr Region of the Ternary Cu–Ti–Zr System. Powder Metall Met Ceram 61, 337–349 (2022). https://doi.org/10.1007/s11106-022-00321-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-022-00321-w