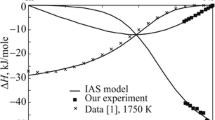
The mixing enthalpies of the La–Ni binary liquid alloys are determined by isoperibol calorimetry in the composition range 0 < x Ni <0.45 at 1430 K and 0.75 < x Ni < 0.80 at 1800 K. The thermodynamic properties of the La–Ni binary liquid alloys are calculated for the entire composition range using the model of ideal associated solutions and reliable published data. The thermodynamic activities of components show negative deviations from the ideal behavior; the mixing enthalpies are characterized by exothermic effects. The minimum mixing enthalpy of the melts is –28.0 ± 0.2 kJ/mol at x Ni = 0.6.










Similar content being viewed by others
References
V. S. Sudavtsova, “Thermodynamic properties of Ni–IIIB metal binary melt,” Metally, No. 6, 119–121 (1999).
S. Watanabe and O. J. Kleppa, “A thermochemical study of liquid and solid alloys (1–x)La+xNi at 1376 K,” J. Chem. Thermodyn., 15, No. 7, 633–644 (1983).
N. P. Lyakishev (ed.), Phase Diagrams of Binary Metal Systems [in Russian], in 3 Vols., Mashinostroenie, Moscow (1998), Vol. 1, p. 992; Vol. 2, p. 1024; Vol. 3, p. 448.
I. V. Nikolaenko and O. V. Vlasova, “Enthalpy of mixing for nickel and lanthanides,” Rasplavy, No. 4, 12–18 (1992).
A. I. Zaitsev, N. E. Zaitseva, N. A. Arutyunyan, and S. V. Dunaev, “Thermodynamic properties and phase equilibria in the Ni–La system: transition of melts in amorphous state,” Zh. Fiz. Khim., 80, No. 5, 787–798 (2006).
J. Dischinger and H.-J. Schaller, “On the constitution and thermodynamics of Ni–La alloys,” J. Alloys Compd., 312, Nos. 1–2, 201–210 (2000).
Z. Du, D. Wang, and W. Zhang, “Thermodynamic assessment of the La–Ni system,” J. Alloys Compd., 264, Nos. 1–2, 209–213 (1998).
T. N. Rezukhina and S. V. Kutsev, “Thermodynamic properties of intermetallics in the La–Ni system,” Zh. Fiz. Khim., 56, No. 1, 1–10 (1982).
X. H. An, Q. F. Gu, J. Y. Zhang, et al., “Experimental investigation and thermodynamic reassessment of La–Ni and La–Ni5–H systems,” Calphad, 40, 48–55 (2013).
M. Ivanov, V. Berezutski, and N. Usenko, “Mixing enthalpies in liquid alloys of manganese with the lanthanides,” J. Mater. Res., 102, 277–281 (2011).
A. T. Dinsdale, “SGTE data for pure elements,” Calphad, 15, 319–427 (1991).
M. I. Ivanov, V. V. Berezutskii, M. O. Shevchenko, et al., “Interaction in europium-containing alloys,” Dop. Akad. Nauk Ukrainy, No. 8, 90–99 (2013).
M. A. Shevchenko, M. I. Ivanov, V. V. Berezutskii, et al., “Thermodynamic properties of Ni–Sc and Ni–Y alloys,” Zh. Fiz. Khim., 88, No. 6, 909–914 (2014).
C. Chatillon-Colinet, H. Diar, J. C. Mathie, et al., “Determination of the enthalpies of formation for LaM and LalTiAl composites by dissolution calorimetry,” Ann. Chim. Fr., 4, No. 8, 657–663 (1979).
K. N. Semenenko, R. A. Sirotina, and A. P. Savchenkova, “Thermochemical study of intermetallics formed in the La–Ni system,” Zh. Fiz. Khim., 53, No. 9, 2373–2374 (1979).
W. N. Hubbard, P. L. Rawlins, P. A. Connick, et al., “The standard enthalpy of formation of LaNi5. The enthalpies of hydriding of LaNi5–x Al x ,” J. Chem. Thermodyn., 15, 785–798 (1983).
A. Pasturel, F. Liautaud, C. Colinet, et al., “Thermodynamic study of the LaNi5–x Cu x system,” J. Less-Common Met., 96, 93–97 (1984).
A. Pasturel, C. Colinet, C. Allibert, et al., “A theoretical and experimental study of the enthalpies of formation of LaNi5-type compounds,” Phys. Status Solidi, 125, 101–106 (1984).
S. V. Kutsev, Thermodynamic Properties of Intermetallic Phases in the Lanthanum–Nickel, Lanthanum–Cobalt, and Praseodymium–Nickel Systems [in Russian], Author’s Abstract of PhD Thesis in Chemistry, Moscow (1984), p. 21.
R. A. Sirotina, Standard Enthalpies of Forming Ni and Co Intermetallics with Lanthanides [in Russian], Author’s Abstract of PhD Thesis in Chemistry, Mosc. Gos. Univ., Moscow (1985), p. 20.
V. N. Denisov, N. M. Klimovskikh, S. M. Kunenko, et al., “Interaction of lanthanum with nickel in electrolysis of chloride solutions,” Izv. Vuz. Tsvet. Metall., No. 4, 77–80 (1986).
C. Colinet, A. Pasturel, A. Percheron-Guegan, and J. C. Achard, “Enthalpies of formation and hydrogenation of La(Ni(1–x)Co x )5 compounds,” J. Less-Common Met., 134, 109–122 (1987).
A. L. Shilov, “Enthalpies of forming some intermetallic compounds,” Zh. Fiz. Khim., 1384–1385 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 55, Nos. 11–12 (512), pp. 105–114, 2016.
Rights and permissions
About this article
Cite this article
Subotenko, P.M., Kudin, V.G., Shevchenko, M.O. et al. Thermodynamic Properties of La–Ni Alloys. Powder Metall Met Ceram 55, 717–725 (2017). https://doi.org/10.1007/s11106-017-9859-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9859-7