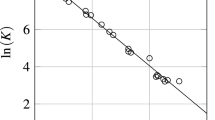
The isothermal sections of the Al2O3–TiO2–Y2O3 phase diagram are constructed for the first time at 1550 and 1400°C. New phases or appreciable homogeneity ranges based on components and binary compounds are not found in the ternary system. Triangulation of the system is determined by the Y2T2O7 phase, which is in equilibrium with compounds Al2TiO5, Y3Al5O12, YAlO3, and Y4Al2O9 and components TiO2 and Al2O3. The system is triangulated into six secondary triangles, in which three-phase eutectics are expected to exist. In five quasibinary sections, two-phase eutectics are likely to exist.





Similar content being viewed by others
Notes
The concentrations are in mol.% here and further in the text.
References
M.-H. Berger and A. Sayir, “Directional solidification of Al2O3–Al2TiO5 system,” J. Eur. Ceram. Soc., 28, 2411–2419 (2008).
V. P. Tarasovskii and E. S. Lukin, “Aluminum titanate—production methods, microstructure, and properties,” Ogneup. Mater., No. 6, 24–31 (1985).
N. F. Toropov, V. P. Barzakovskii, V. V. Lapin, and N. N. Kurtzeva, Phase Diagrams of Silicate Systems: Handbook. First Edition. Binary Systems [in Russian], Nauka, Leningrad (1969), Vol. 1, p. 822.
Nobuyasu Mizutani, Yo Tajima, and Masanori Kato, “Phase relations in the system Y2O3–TiO2,” J. Am. Ceram. Soc., 59, 168 (1976).
A. J. Feighery, J. T. S. Irvine, D. P. Fagg, and A. Kaiser, “Phase relations at 1500°C in the ternary system ZrO2–Y2O3–TiO2,” J. Solid State Chem., No. 143, 273–276 (1999).
T. A. Schaedler, O. Fabrichnaya, and C. G. Levi, “Phase equilibria in the TiO2–YO1.5–ZrO2 system,” J. Eur. Ceram. Soc., 28, 2509–2520 (2008).
Weiping Gong, Dajian Li, Zhongshen Chen, et al., “Phase equilibria of the TiO2–Y2O3 system,” CALPAD, 33, 624–627 (2009).
N. A. Toropov, I. A. Bondar, F. Ya. Galakhov, et al., “Phase equilibria in the yttria–alumina system,” Izv. Akad. Nauk SSSR, Ser. Khim., No. 7, 1158–1164 (1964).
D. Viechnicki and F. Schmidt, “Eutectic solidification in the system Al2O3/Y3Al5O12,” J. Mater. Sci., 4, No. 1, 84–88 (1969).
J. L. Caslavsky and D. J. Viechnicki, “Melting behavior and metastability of yttrium aluminum garnet (YAG) and YAlO3 determined by optical differential thermal analysis,” J. Mater. Sci., 15, No. 7, 1709–1718 (1980).
I. A. Bondar, L. N. Koroleva, and E. T. Bezruk, “Physicochemical properties of yttrium aluminates and gallates,” Izv. Akad. Nauk SSSR. Neorg. Mater., 20, No. 2, 257–261 (1984).
B. Cocayne and B. A. Lent, “Complexity in the solidification behavior of molten Y3Al5O12,” J. Cryst. Growth, 46, No. 3, 371–378 (1979).
B. Cocayne, “The uses and enigmas of the Al2O3–Y2O3 phase system,” J. Less-Common Met., 114, No. 1, 199–206 (1985).
T. Mah and D. Petry, “Eutectic composition in the pseudobinary of Y4Al2O9 and Y2O3,” J. Am. Ceram. Soc., 75, No. 7, 2006–2009 (1992).
R. S. Hay, “Kinetics and deformation during the reaction of yttrium-aluminum perovskite and alumina to yttrium–aluminum garnet,” J. Am. Ceram. Soc., 77, No. 6, 1473–1485 (1994).
M. Gervais and A. Douy, “Solid phase transformation and melting of the compounds Ln4Al2O9 (Ln = Gd, Dy, Y),” Mater. Sci. Eng. B, 38, Nos. 1–2, 118–121 (1996).
M. Shimada and H. Yamane, “Martensitic transformation of Y4Al2O9,” Ceram. Eng. Sci. Proc., 21, No. 4, 455–460 (2000).
O. Fabrichnaya, H. J. Seifert, T. Ludwig, et al., “The assessment of thermodynamic parameters in the Al2O3–Y2O3 system and phase relations in the Y–Al–O system,” Scand. J. Met., 30, No. 3, 175–183 (2001).
Y. Mizutani, H. Yasuda, I. Ohnaka, et al., “Phase selection of the Al2O3–Y2O3 system controlled by nucleation,” Mater. Trans., 42, No. 2, 238–244 (2001).
M. Mizuno and T. Noguchi, “Y2O3–Al2O3 system. I. Formation of compounds in Y2O3–Al2O3 system,” Rep. Gov. Ind. Res. Inst. Nagoya, 16, 171–178 (1967).
M. Mizuno and T. Noguchi, “Y2O3–Al2O3 system. II. Liquidus curve measurement in the Y2O3–Al2O3 system,” Rep. Gov. Ind. Res. Inst. Nagoya, 16, 210–216 (1967).
S. M. Lakiza and L. M. Lopato, “Stable and metastable phase relations in the system alumina–zirconia–yttria,” J. Am. Ceram. Soc., 80, No. 4, 893–902 (1997).
J. S. Abell, I. R. Harris, B. Cocayne, et al., “An investigation of phase stability in the Y2O3–Al2O3 system,” J. Mater. Sci., 9, No. 4, 527–537 (1974).
G. T. Adylov, G. V. Voronov, E. P. Mansurova, et al., “The Y2O3–Al2O3 system above 1473 K,” Zh. Neorg. Khim., 33, No. 7, 1867–1869 (1988).
H. Yamane, M. Omori, A. Okubo, et al., “High-temperature phase transition of Y4Al2O9,” J. Am. Ceram. Soc., 76, No. 9, 2382–2384 (1993).
H. Yamane, M. Shimada, and B. A. Hunter, “High-temperature neutron diffraction study of Y4Al2O9,” J. Solid State Chem., 141, No. 2, 466–474 (1998).
L. E. Matson, R. S. Hay, and T. Mah, “Characterization of alumina/yttrium-aluminum garnet and alumina/yttrium-aluminum perovskite eutectics,” Ceram. Eng. Sci. Proc., 11, Nos. 7–8, 995–1003 (1990).
H. Yasuda, I. Ohnaka, Y. Mizutani, et al., “Selection of eutectic system in Al2O3–Y2O3 ceramics,” Sci. Technol. Adv. Mater., 2, 67–71 (2001).
H. Yasuda, Y. Mizutani, I. Ohnaka, et al., “Undercooled melt formation and shaping of alumina–yttrium aluminum garnet eutectic ceramics,” J. Am. Ceram. Soc., 86, No. 10, 1818–1820 (2003).
H. Yasuda, I. Ohnaka, Y. Mizutani, et al., “Solidification and shape casting of Al2O3–YAG eutectic ceramics from the undercooled melt produced by melting Al2O3–YAP eutectics,” Sci. Technol. Adv. Mater., 5, No. 1–2, 207–217 (2004).
M. Vlasova, M. Kakazey, P. A. Márquez Aguilar, et al., “Directed laser processing of compacted powder mixtures Al2O3–TiO2–Y2O3,” Sci. Sintering, 45, 247–259 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 55, Nos. 11–12 (512), pp. 82–92, 2016.
Rights and permissions
About this article
Cite this article
Tishchenko, Y.S., Lakiza, S.M., Red’ko, V.P. et al. Isothermal Sections of the Al2O3–TiO2–Y2O3 Phase Diagram at 1550 and 1400°C. Powder Metall Met Ceram 55, 698–706 (2017). https://doi.org/10.1007/s11106-017-9857-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9857-9