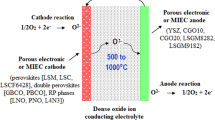
The solid oxide fuel cell is a promising element that efficiently converts fuel chemical energy into electrical and thermal ones. The paper offers a brief overview of the ways for enhancing the properties of the anode by improving its composition and structure. The composition and structure of the anode are responsible for the electrochemical oxidation of fuel. The ability of the solid oxide fuel cell to perform reliably with different fuel types (H 2 , C n H m , CO) is one of the key requirements for its further commercialization.




Similar content being viewed by others
References
S. C. Singhal and K. Kendall, High-temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications, Elsevier, Oxford, U.K. (2003), p. 406.
F. Tietz, H.-P. Buchkremer, and D. Stover, “Components manufacturing for solid oxide fuel cells,” Solid State Ionics, 152–153, 373–381 (2002).
R. J. Gorte and J. M. Vohs, “SOFC anodes for the direct electrochemical oxidation of hydrocarbons,” J. Catal., 216, 477–486 (2003).
H. S. Spasil, Electrical Device Including Nickel-Containing Stabilized Zirconia Electrode, US Patent 3.558.360, October 30, 1964; modified November 2, 1967; publ. March 31 (1970).
K. C. Wincewicz and J. S. Cooper, “Taxonomies of SOFC material and manufacturing alternatives,” J. Power Sources, 140, 280–296 (2005).
T. Kawada and J. Mizusaki, “Current electrolytes and catalysts,” in: W. Vielstich et al. (eds.), Handbook of Fuel Cells—Fundamentals, Technology and Application, Vol. 4, Fuel Cell Technology and Applications, Wiley and Sons, Chichester, England (2003), p. 987.
J. Molenda, K. Swierczek, and W. Zajac, “Functional materials for the IT-SOFC,” J. Power Sources, 173, 657–670 (2007).
N. Q. Minh and T. Takahashi, Science and Technology of Ceramic Fuel Cells, Elsevier Science, Amsterdam (1995), p. 356.
S. T. Aruna, M. Muthuraman, and K. C. Patil, “Synthesis and properties of Ni–YSZ cermet anode material for solid oxide fuel cells,” Solid State Ionics, 111, 45–51 (1998).
M. Mori, T. Yamamoto, H. Itoh, et al., “Thermal expansion of nickel–zirconia anodes in solid oxide fuel cell during fabrication and operation,” J. Electrochem. Soc., 145, 1374–1381 (1998).
P. Duran, J. Tartaj, and F. Capel, “Processing and characterization of nickel oxide/zirconia composite prepared by polymeric complex solution synthesis,” J. Eur. Ceram. Soc., 23, 2125–2133 (2003).
S. Kim, H. Moon, and S. Hyun, “Ni–YSZ cermet anode fabricated from NiO–YSZ composite powder for high-performance and durability of solid oxide fuel cells,” Solid State Ionics, 178, 1304–1309 (2007).
T. Kim, G. Liu, M. Boaro, et al., “A study of carbon formation and prevention in hydrocarbon-fueled SOFC,” J. Power Sources, 155, 231–238 (2006).
M. L. Toebes, J. H. Bitter, and A. J. Van Dillen, “Impact of the structure and reactivity of nickel particles on the catalytic growth of carbon nanofibers,” Catal. Today, 76, 33–42 (2002).
K. Nikooyeh, R. Clemmer, V. Alzate-Restrepo, et al., “Effect of hydrogen on carbon formation on Ni/YSZ composites exposed to methane,” Appl. Catal. A: Gen., 347, 106–111 (2008).
H. Timmermann, W. Sawady, and D. Campbell, “Coke formation and degradation in SOFC operation with a model reformate from liquid hydrocarbons,” J. Electrochem. Soc., 155, No. 4, B356–B359 (2008).
Y. B. Lin, Z. L. Zhan, J. Liu, et al., “Direct operation of solid oxide fuel cells with methane fuel,” Solid State Ionics, 176, 1827–1835 (2005).
M. D. Gross, J. M. Vohs, and R. J. Gorte, “Recent progress in SOFC anodes for direct utilization of hydrocarbons,” J. Mater. Chem., 17, 3071–3077 (2007).
H. Kan and H. Lee, “Sn-doped Ni/YSZ anode catalysts with enhanced carbon deposition resistance for an intermediate temperature SOFC,” Appl. Catal. B: Environ., 97, 108–114 (2010).
L. Jia, X. Wanga, B. Hua, et al., “Computational analysis of atomic C and S adsorption on Ni, Cu, and Ni–Cu SOFC anode surfaces,” Int. J. Hydrogen Energy, 37, 11941–11945 (2012).
C. Lu, S. An, W. L. Worrell, et al., “Development of intermediate-temperature solid oxide fuel cells for direct utilization of hydrocarbon fuels,” Solid State Ionics, 175, 47–50 (2004).
R. J. Gorte, S. Park, J. M. Vohs, et al., “Anodes for direct oxidation of dry hydrocarbons in a solid-oxide fuel cell,” Adv. Mater., 12, 1465–1469 (2000).
C. M. Grgicak, M. M. Pakulska, and J. S. O’Brien, “Synergistic effects of Ni1–x Co x –YSZ and Ni1–x Cu x –YSZ alloyed cermet SOFC anodes for oxidation of hydrogen and methane fuels containing H2S,” J. Power Sources, 183, 26–33 (2008).
E. W. Park, H. Moon, M. S. Park, et al., “Fabrication and characterization of Cu–Ni–YSZ SOFC anodes for direct use of methane via Cu-electroplating,” Int. J. Hydrogen Energy, 34, 5537–5545 (2009).
Y. Matsuzaki and I. Yasuda, “The poisoning effect of sulfur-containing impurity gas on a SOFC anode. I. Dependence on temperature, time, and impurity concentration,” Solid State Ionics, 132, 261–269 (2000).
S. Zha, Z. Cheng, and M. Liu, “Sulfur poisoning and regeneration of Ni-based anodes in solid oxide fuel cells,” J. Electrochem. Soc., 154, No. 2, B201–B206 (2007).
K. Sasaki, K. Susuki, A. Iyoshi, et al., in: S. Singhal and J. Mizusaki (eds.), Proc. Solid Oxide Fuel Cells IX, The Electrochemical Society, Inc., Quebec (2005), Vol. 2, pp. 1267–1274.
T. K. Shashkova, M. R. Kantserova1, V. I. Chedryk, et al., “Methane oxidative conversion over the composites of Y- and Sc-stabilized zirconia based,” Pol. J. Chem., 82, 371–376 (2008).
H. He, R. J. Gorte, and J. M. Vohs, “Highly sulfur tolerant Cu–Ceria anodes for SOFCs,” Electrochem. Solid-State Lett., 8, No. 6, A279–A280 (2005).
J. W. Fergus, “Oxide anode materials for solid oxide fuel cells,” Solid State Ionics, 177, 1529–1541 (2006).
M. K. Rath, B. G. Ahn, and B. H. Choi, “Effects of manganese substitution at the B-site of lanthanum-rich strontium titanate anodes on fuel cell performance and catalytic activity,” Ceram. Int., 39, 6343–6353 (2013).
G. Pudmicha, B. A. Boukampb, M. Gonzalez-Cuencab, et al., “Chromite/titanate based perovskites for application as anodes in solid oxide fuel cells,” Solid State Ionics, 135, 433–438 (2000).
Y. H. Huang, R. I. Dass, Z. L. Xing, et al., “Double perovskites as anode materials for solid-oxide fuel cells,” Science, 312, 254–257 (2006).
D. W. Dees, T. D. Claar, T. E. Ealser, et al., “Conductivity of porous Ni/ZrO2–Y2O3 cermets,” J. Electrochem. Soc., 134, 2141–2146 (1987).
O. Vasylyev, I. Brodnikovskyi, M. Brychevskyi, et al., “NiO–10Sc1CeSZ anode: structure and mechanical behavior,” in: N. P. Bansal (ed.), Proc. Ceramic Engineering and Science: Advances in Solid Oxide Fuel Cells III, Wiley (2007), Vol. 28, No. 4, pp. 361–337.
F. Tietz, F. J. Dias, D. Simwonis, et al., “Evaluation of commercial nickel oxide powders for components in solid oxide fuel cells,” J. Eur. Ceram. Soc., 20, 1023–1034 (2000).
X. H. Fang, G. Y. Zhu, and C. R. Xia, “Synthesis and properties of Ni–SDC cermets for IT-SOFC anode by co-precipitation,” Solid State Ionics, 168, No. 1–2, 31–36 (2004).
A. Ringuedé, D. Bronine, and J. R. Frade, “Assessment of Ni/YSZ anodes prepared by combustion synthesis,” Solid State Ionics, 146, No. 3–4, 219–224 (2002).
S. K. Pratihar, A. Dassharma, and H. S. Maiti, “Processing microstructure property correlation of porous Ni–YSZ cermets anode for SOFC application,” Mater. Res. Bull., 40, No. 11, 1936–1944 (2005).
R. F. Martins, M. C. Brant, R. M. Paniago, et al., “Synthesis and characterization of NiO–YSZ for SOFCs,” Mater. Res. Bull., 44, No. 2, 451–456 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 54, Nos. 3–4 (502), pp. 49–59, 2015.
Rights and permissions
About this article
Cite this article
Brodnikovskii, E.M. Solid Oxide Fuel Cell Anode Materials. Powder Metall Met Ceram 54, 166–174 (2015). https://doi.org/10.1007/s11106-015-9694-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9694-7