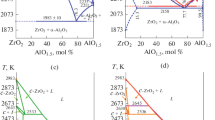
For more complete description of the Al2O3–HfO2–Gd2O3 phase diagram, three vertical sections are constructed in a wide temperature and composition range. The Gd2O3 and HfO2 corner bisectors explain the mechanisms of Gd2O3 phase transformations, C ⇆ X ⇆ H ⇆ A ⇆ B, and interactions in the HfO2 corner of the system. The isopleth at 15 mol.% HfO2 (15H) shows the Al2O3–HfO2–Gd2O3 constitution in Al2O3- and Gd2O3-rich regions.





Similar content being viewed by others
References
S. M. Lakiza, Ya. S. Tishchenko, and L. M. Lopato, “Projections of the liquidus and solidus surfaces of the Al2O3–HfO2–Gd2O3 phase diagram,” Powder Metall. Met. Ceram., 50, No. 7–8, 429–441 (2011).
S. M. Lakiza, Ya. S. Tishchenko, V. P. Red’ko, and L. M. Lopato, “The Al2O3–HfO2–Gd2O3 phase diagram I. Isothermal sections at 1250 and 1650°C,” Powder Metall. Met. Ceram., 51, No. 1–2, 87–92 (2012).
L. M. Lopato, A. V. Shevchenko, and G. I. Gerasimyuk, “The HfO2–Al2O3 system,” Izv. AN SSSR. Neorg. Mater., 12, No. 9, 1623–1626 (1976).
P. Duran, “Phase relationships in the hafnia–gadolinia system,” Ceramurg. Int., 3, No. 4, 137–140 (1977).
A. V. Shevchenko, L. M. Lopato, and L. V. Nazarenko, “Systems of HfO2 with samarium, gadolinium, terbium, and dysprosium oxides at high temperatures,” Izv. AN SSSR. Neorg. Mater., 20, No. 11, 1862–1866 (1984).
P. P. Budnikov, V. I. Kushakovskii, and V. S. Belevantsev, “Study of the Gd2O3–Al2O3 and Sm2O3–Al2O3 systems,” Dokl. AN SSSR, 165, No. 5, 1075–1077 (1965).
M. Mizuno, T. Yamada, and T. Noguchi, “Phase diagrams of the systems Al2O3–Eu2O3 and Al2O3–Gd2O3 at high temperatures,” J. Ceram. Soc. Jap., 85, No. 11, 543–549 (1977).
M. Gervais and A. Douy, “Solid phase transformation and melting of the compounds Ln4Al2O9 (Ln = Gd, Dy, Y),” Mater. Sci. Eng., B38, No. 1–2, 118 (1996).
J. Coutures and J. P. Coutures, “Etude par rayons X a haute temperature des transformations polymorphiques des perovskites LnAlO3 (Ln = element lanthanidique),” J. Solid State Chem., 52, No. 2, 95–100 (1984).
T. Shishido, K. Okamura, and S. Yajima, “Gd3Al5O12 phase obtained by crystallization of amorphous Gd2O3 × 5/3Al2O3,” J. Am. Ceram. Soc., 61, No. 7–8, 373–375 (1978).
N. N. Matyushenko, É. P. Shevyakova, E. V. Lifshitz, et al., “Crystal structure and some properties of gadolinium aluminate Gd3Al5O12,” Zh. Neorg. Chim., 30, No. 7, 1654–1657 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
L. M. Lopato (Deceased).
Translated from Poroshkovaya Metallurgiya, Vol. 51, No. 5–6 (485), pp. 84–92, 2012.
Rights and permissions
About this article
Cite this article
Tishchenko, Y.S., Lakiza, S.M. & Lopato, L.M. The Al2O3–HfO2–Gd2O3 phase diagram. II. Vertical sections. Powder Metall Met Ceram 51, 316–322 (2012). https://doi.org/10.1007/s11106-012-9434-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-012-9434-1