Abstract
Growth of Arabidopsis is controlled by the activity of a set of bHLH and bZIP transcription factors of which phytochrome interacting factor4 (PIF4), BRASSINAZOLE-RESISTANT 1 (BZR1), and elongated hypocotyl 5 (HY5) have been most extensively studied. Defense responses are controlled by a set of MYC transcription factors of which MYC2 is best characterized. Moreover, hundreds of additional proteins (here named co-factors) have been identified which (in)directly may affect the expression or activity of these TFs. Thus, regulation of expression of genes encoding these co-factors becomes an integral part of understanding the molecular control of growth and defense. Here, we review RNA-seq data related to PIF, BZR1, HY5, or MYC activity, which indicate that 125 co-factor genes affecting PIFs, HY5, BZR1, or MYCs are themselves under transcriptional control by these TFs, thus revealing potential feedback regulation in growth and defense. The transcriptional feedback on co-factor genes related to PIF4, BZR1, and MYC2 by PIFs, BZR1, or MYCs, mostly results in negative feedback on PIF4, BZR1, or MYC2 activity. In contrast, transcription feedback on co-factor genes for HY5 by HY5 mostly results in positive feedback on HY5 activity. PIF4 and BZR1 exert a balanced regulating of photoreceptor-gene expression, whose products directly or indirectly affect PIF4, HY5, and MYC2 protein stability as a function of light. Growth itself is balanced by both multiple positive and multiple negative feedback on PIF4 and BZR1 activity. The balance between growth and defense is mostly through direct cross-regulation between HY5 and MYC2 as previously described, but also through potential transcriptional feedback on co-factor genes for MYC2 by PIF4, BZR1, and HY5 and through transcriptional feedback of co-factors for PIF4 and BZR1 by MYC2. The interlocking feed-forward and feed-backward transcriptional regulation of PIF4, BZR1, HY5, and MYC2 co-factors is a signature of robust and temporal control of signaling related to growth and defense.
Similar content being viewed by others
Introduction
Key Transcription Factors Acting in Growth and Defense in Arabidopsis: PIFs, BZR1, HY5, and MYCs
The molecular components involved in cell elongation and defense responses in plants have most extensively been studied in the model plant Arabidopsis. Numerous transcription factors (TFs) are involved in growth control, of which the basic helix-loop-helix transcription factors phytochrome interacting factors (PIFs) and the brassinolide activated transcription factor protein BRASSINAZOLE-RESISTANT 1 (BZR1) and its homolog BRI1-EMS suppressor 1 (BES1) have been shown to be key factors in stimulating growth under different environmental conditions (darkness, heat, shade) (Leivar et al. 2008; Quint et al. 2016, Li et al. 2020a). In contrast, suppression of growth in the light is mainly by the basic leucine zipper transcription factors elongated hypocotyl 5 (HY5) and its homolog (HYH) (Gangappa and Botto 2016). Table S1 shows the number of publications for TFs listed in combination with terms related to the environmental conditions of darkness, light, heat, shade, or defense, represented by the terms skotomorphogenesis, photomorphogenesis, thermomorphogenesis, and defense, respectively. The inventory shows that from the PIFs, PIF4 is the most representative/studied TF; from the brassinolide (BR) regulated TFs, BZR1 has been most extensively studied; and from the growth-suppressing TFs, HY5 has most extensively been studied (Table S1). Therefore, the focus here is on the transcriptional regulation of PIF4, BZR1, and HY5 during growth. Biotic stress responses triggered by the Jasmonic acid signaling pathway result in activation of MYC transcription factors, which target defense-related genes. Of the different defense-related MYC genes (MYC2, MYC3, and MYC4), specifically the action of MYC2 is key and has been best studied in Arabidopsis (Table S1) (Fernandez-Calvo et al. 2011). Therefore, in this study on transcriptional regulation for defense responses, the focus is on MYC2. Our aim is to summarize the literature on transcriptional regulation of the main transcription factors in growth and defense and use available omics-data sets to uncover previously unnoticed potential for transcriptional positive and negative feedback regulation.
Different Levels of Potential Feedback on PIF4, BZR1, HY5, or MYC2 Activity
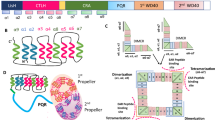
When PIFs, BZR1, HY5, and MYCs are key TFs in growth and defense, the TFs that act on the promoter of the genes encoding these key TFs are expected to be higher order TFs that regulate growth and defense, unless these key TFs play a dominant role in regulating their own expression. Indeed, the genes for each of these key TFs have sequences in their promoter for potential self-binding, indicating the potential for auto-feedback (as shown as an example for PIF4, Fig. 1A, direct feedback a). In addition, each of the key TFs may affect the expression of other TF genes that bind to the promoter of the key TFs (as shown for PIF4, Fig. 1A, in-direct feedback b). While transcription and translation may determine the level of key TF proteins, and thus the potential capacity for key TF activity, the actual key TF activity is determined by additional factors acting at the post-transcriptional level (reviewed in (Koini et al. 2009, Fernandez-Calvo et al. 2011, Gangappa and Botto 2016, Ibanez et al. 2018, Martinez et al. 2018; Qiu 2020)). The genes encoding these co-factors may themselves be (partially) under the control of the key TF itself, thus forming potential additional indirect feedback on each key TF (as shown for PIF4 in Fig. 1A, feedback c. In the same way, three levels of potential feedback on BZR1, HY5, and MYC2 may be drawn).
Multiple potential (in)direct feedback on key TF activity. A Potential (in)direct feedback on key TF activity is illustrated for PIF4, but a similar scheme can be drawn for the other key TFs. PIF4 gene (coding sequence and promoter), PIF4 related co-factor gene (coding sequence and promoter), and genes for TF X acting on PIF4 (coding sequence and promoter) are indicated by line (promoter) and rectangle (coding sequence). PIF4 protein, PIF4-related co-factor protein, and TF X protein are indicated by an oval. PIF4 has binding sites for PIF4 in its own promoter, indicating a potential role for PIF4 in its own transcription (potential direct auto-feedback regulation loop (a). Multiple TFs are involved in the transcription of PIF4, of which the corresponding genes may be targeted by PIF4 (potential indirect transcriptional feedback loops (b). In addition, multiple co-factors may act on the activity/stability of PIF4 or TF X acting on PIF4 (dashed arrow). The genes encoding for these co-factors may be target of PIF4 (potential indirect autofeedback regulation on PIF4: (c). Potential transcriptional feedback indicated by the solid black arrows, potential post-transcriptional feedback on PIF4 or TFX protein indicated by a dashed black arrow. Similar feedback interaction schemes may be drawn for BZR1, HY5, and MYC2. B Different types of co-factors acting on PIF4, BZR1, HY5, or MYC2 protein. Left: factors with positive feedback on the key TFs (Green arrows), Right: factors with negative feedback on the key TFs (Red arrows). TF activators and repressors: Proteins that interact with the key TFs in transcription complexes to either stimulate or block transcriptional activity. Chromatin: chromatin modifying factors. Proteasome: components of multi-protein complexes that target proteins for degradation. Sequestering: proteins that interact with the key TFs to prevent their transcriptional activity
The literature describes different types of co-factors that affect either PIF4, BZR1, HY5 or MYC2 activity (Fig. 1B). Our inventory yielded 102 co-factor genes for PIF4 (Table S2), 93 co-factor genes for BZR1 (Table S3), 36 co-factor genes for HY5 (Table S4) and 57 co-factor genes for MYC2 (Table S5). Tables S2-S5 also give a brief description of each co-factor acting on PIF4, BZR1, HY5, or MYC2 and refers to the individual papers describing the experimental conditions under which these factors affect the TF activity. Among these co-factors are other TFs (e.g., certain clock genes) that may either aid or block the transcriptional activity of PIF4, BZR1, HY5, or MYC2. Some co-factors are part of a gene family such as the family of JAZ repressors (12 members), or the family of DELLA proteins (5 members) with sequestering activity. While the interaction with our key TFs may only have been shown for some family members, here all gene family members are considered potential co-factor (see Tables S2-5). The activity of BZR1 and MYC2 is coupled to brassinosteroid (BR) biosynthesis and signaling and Jasmonic acid (JA) biosynthesis and signaling, respectively. Therefore, genes involved in BR biosynthesis and signaling were included as potential positive acting co-factor genes for BZR1 and JA biosynthesis genes and signaling were included as potential additional positive acting co-factor genes for MYC2 (see Tables S2-5). The activity of phosphorylated BZR1 and PIFs is effectuated by the binding of 14–3-3 proteins (Gampala et al. 2007, Wang et al. 2011, Wang et al. 2013a, b, Srivastava, Srivastava et al. 2020). Binding of 14–3-3 to phosphorylated BZR1 results in cytosolic retention of BZR1 and thus 14–3-3 proteins are negative co-factors for BZR1 activity in the nucleus. Also, for PIF proteins, 14–3-3 binding results in reduced PIF activity (Adams et al. 2014, Camoni et al. 2018, Huang et al. 2018). Therefore, the set of 14–3-3 genes is included as potential negative acting co-factor genes for both PIF4 and BZR1. In addition, co-factors may be part of protein complexes involved in the specific degradation of one of the four TF proteins. The summary in Fig. 1B shows the potential positive (green arrows) or negative (red arrows) feedback of the different types of co-factors on either PIF4, BZR1, HY5, or MYC2. The regulation of the genes encoding the numerous co-factors that modulate PIF4, BZR1, HY5, or MYC2 protein activity, by these same key TFs, form the potential of indirect feedback as described in Fig. 1A (feedback c). This potential for feedback regulation through co-factor genes has not been studied systematically and is here determined from available transcriptome data related to the transcriptional activity of PIFs, BZR1, HY5, or MYCs (see below).
Potential for Auto-Feedback and Indirect Feedback on PIFs, BZR1, HY5, and MYCs
The inventory of validated TFs binding to promoters of PIF4, BZR1, HY5, and MYC2 from ChIP-seq experiments as available at PlantPan3.0 show 34 TFs with binding to the PIF4 promoter, 36 TFs with binding to the BZR1 promoter, 26 TFs with binding to the HY5 promoter and 32 TFs with binding to the MYC2 promoter (Fig. 2A). Actually, no less than 16 TFs share binding to each of the four promoters, one of which is PIF4 itself (Fig. 2A). Although this indicates a potential central role for PIF4 in the expression of all four selected TFs, no evidence is found in RNA-seq data that PIF4 or PIFs are limiting in the transcription of BZR1, HY5, or MYC2, suggesting a redundant function of PIFs acting on these promoters. As described above, there is an overlap for co-factors that act on PIF4, BZR1, MYC2, or HY5 protein (Fig. 2B). This suggests a potential for coordinated activity between these four key TFs as effectuated by co-factor action. (Venn diagram of Tables S2-S5: Fig. 2B). PIF4 and BZR1 share 36 co-factor genes. PIF4 and MYC2 share 27 co-factor genes, BZR1 and MYC2 share 12 co-factor genes, and HY5 and MYC2 only share 8 co-factor genes (Fig. 2B). The inventory indicates PIF4 as an indirect co-factor for BZR1 and HY5 activity, BZR1 as co-factor for its own activity and PIF4 activity and MYC2 is a direct co-factor for its own activity and indirect co-factor for PIF4 and HY5 activity (Fig. 2B). HY5, COP1, and photoreceptors PHYB, CRY1, and CRY2 have a central position as shared co-factors for all four selected TFs. HY5 can affect PIF4 by competitive binding of HY5 at PIF4 target sites (Delker et al. 2014; Gangappa and Kumar 2017). HY5 potentially affects BZR1 by sequestering de-phosphorylated (active) BZR1/BES1 (Li and He 2016), HY5 potentially affects its own protein stability by activating COP1 transcription under UV light (Huang et al. 2012), and HY5 represses transcription of MYC2 (Chakraborty et al. 2019). COP1 is required for the stabilization of PIF proteins in the dark (Gangappa and Kumar 2017) and COP1 interacts with and degrades the phosphorylated (inactive) BZR1 protein. While this decreases the total pool of BZR1 protein, this may increase the chance of active BZR1 homodimer formation (Kim et al. 2014). COP1 has a negative effect on HY5 protein levels, as it targets HY5 and the positive TFs for HY5 transcription (BBX, LAF1, HFR1) for degradation (Saijo et al. 2003; Gangappa and Botto 2016). Under UV light, COP1 stabilizes GBF1, a negative acting TF for HY5 transcription, but also HYH protein, a positive acting TF for HY5 transcription (Gangappa and Botto 2016). Finally, COP1 is also required for the degradation of MYC2 in the dark (Chico et al. 2014). The red-light activated PHYBPfr interacts with PIF proteins and targets them for degradation (Huq and Quail 2002; Lorrain et al. 2008). Because BZR1 and PIF4 share common co-factor target genes, the effect of PHYB on PIF4 protein levels indirectly affects the downstream activity of BZR1 (Kim et al. 2014). PHYBPfr prevents the interaction of HY5 protein with the COP1/SPA complex and thus results in the stabilization of HY5 protein (Lu et al. 2015). Finally, PHYBPfr increases MYC2 protein stability, but at the same time enhances the stability of JAZ repressor proteins, resulting in suppression of MYC2 target gene expression (Chico et al. 2014). Figure 1B summarizes the different types of co-factors that may have either positive or negative effect on PIF4, BZR1, HY5, or MYC2 protein activity. Co-factors that influence PIF4, BZR1, HY5, or MYC2 may vary from TFs, kinases, phosphatases, components of protein complexes involved in protein degradation and proteins that can sequester these TFs or enzymes involved in hormone biosynthesis that leads to activation of BZR1 or MYC2.
Overlap in transcription factors and co-factors acting on PIF4, BZR1, HY5, or MYC2. A Overlap in validated TFs binding to the promoter of PIF4, BZR1, HY5, or MYC2 (data from PlantPan3.0). Positions of PIF1, PIF4 and PIF5 are indicated in red. Positions of MYC2, HY5, and BZR1 are indicated in green: data from (Lee et al. 2007, Yu et al. 2011, Binkert et al. 2014, Oh et al. 2014, You et al. 2019). PIF4 interacts with the promoter of both PIF4, BZR1, HY5, and MYC2. However, PIF4 alone does not affect the expression of these genes (see Table S2). The 15 additional TFs binding to the promoter of both PIF4, BZR1, HY5, and MYC2 are ABF1, ABF3, ANAC032, AT5G04760, BBM, GBF2, HB5, HB6, LEC1, MYB44, NFYC2, RD26, ZAT6. For a description of the action of these co-factors see Table S2-5. Although DNA ChIP experiments do not show the binding of PIF3 to any of the four key TF genes, PIF3 is involved in the expression of PIF4 and HY5. B Overlap in co-factors acting on PIF4, BZR1, HY5, or MYC2 protein (see Table S2-S5) The positions of PIFs, MYCs, BZR1, and HY5 are indicated in red
Omics Meta-Analysis to Uncover Potential Feedback Regulation on PIF4, BZR1, HY5, and MYC2 Activity
The inventory of TFs and co-factors acting on PIF4, BZR1, HY5, and MYC2 was used to uncover the potential for feedback regulation. PIF4, BZR1, HY5, and MYC2 may feedback on their own activity through transcriptional feedback on the genes encoding co-factors that act on PIF4, BZR1, HY5, or MYC2 protein. The potential for (in)direct auto-feedback on the four TFs through their respective co-factor genes is determined from available ChIP-seq sequence data using tagged PIFs (Oh et al. 2012; Pfeiffer et al. 2014), BZR1 (Oh et al. 2012, Oh et al. 2014) or BES1 (Yu et al. 2011), HY5 (Lee et al. 2007), or MYC2 protein (You et al. 2019). Whether binding of the TF to the promoter of its co-factor gene actually results in transcriptional regulation was determined from a comparison of the transcriptome in WT and the different pif mutants (Leivar et al. 2012; Oh et al. 2012; Huai et al. 2018) bzr1-1D mutant (Oh et al. 2014), hy5 mutant (Zhao et al. 2019), or myc2/3/4 triple mutant (Van Moerkercke et al. 2019). When PIF4 binds to the promoter of a TF-gene or co-factor gene involved in regulating PIF4 transcription or activity, this is counted as a potential for transcriptional auto-feedback regulation by PIF4 (idem for BZR1, HY5, and MYC2). In addition to PIF4 ChIP-seq data, also ChIP-seq binding data by PIF1, PIF3, or PIF5 protein is included in the inventory because of potential redundancy by other PIFs in co-regulation of PIF4 target genes. The contribution of just PIF4 in regulating transcription of a target gene may not be noticeable in a pif4 single-mutant background. Therefore, transcriptome analysis of the pif1/3/4/5 quadruple (pifq) mutant was included in the transcriptome analysis (Pfeiffer et al. 2014). Altered expression of PIF4-related co-factor genes in pifq mutant background was treated here as an indication of potential feedback regulation by PIF4. Similarly, the contribution of only MYC2 in regulating transcription of a target gene may not be noticeable in a myc2 single-mutant background. Therefore, regulation of MYC2 co-factor genes by MYC2 was derived from the comparison of the transcriptome in myc2/3/4 triple mutant versus WT (Schweizer et al. 2013; Van Moerkercke et al. 2019) or 35S-MYC2 lines versus WT (Chini et al. 2007). Since the full knock-out mutant for BZR1 is lethal, transcriptional regulation by BZR1 was from comparing WT with the bzr1-1D mutants, in which BZR1 protein is constitutively active due to point mutation in the phosphorylation site (Wang et al. 2002) and from WT plants treated with an inhibitor of endogenous BR biosynthesis which prevents activation of endogenous BZR1/BES1 and from the comparison of pifq/bzr1-1D versus pifq (Oh et al. 2012; Oh et al. 2014).
Using a comprehensive inventory of relevant ChIP-seq and RNA-seq data sets the potential for direct and indirect feedback on the four selected TFs was determined (see Supplement Methods). Although some feedback on the four selected TFs has been described in the literature, the inventory identifies numerous new potential feedbacks on PIF4, BZR1, HY5, and MYC2 at (post)-transcriptional level and indicates for each TF both positive and negative feedback that may help to maintain homeostasis. Tables 1, 2, 3, and 4 list all the co-factor genes for which either binding to the promoter or transcriptional regulation by PIF4, BZR1, HY5, or MYC2 was identified. In each table, the transcriptional regulation of co-factor genes that results in negative feedback on PIF4, BZR1, HY5, or MYC2 transcription/activity respectively is indicated in red, while transcriptional regulation of co-factor genes that result in positive feedback on PIF4, BZR1, HY5, or MYC2 transcription/activity respectively is indicated in green. Overall, there is little overlap between the binding of PIF4, BZR1, HY5, and MYC2 to their respective co-factor genes (ChIP-seq data) and regulation of their own co-factor genes (RNA-seq data), confirming a general lack of overlap between the ChIP-seq and RNA-seq data sets that have been observed before (Pfeiffer et al. 2014). However, since here data was collected from different publications, the lack of overlap in ChIP-seq and RNA-seq data may also be due to different experimental conditions used in ChIP-seq and RNA-seq experiments.
For discussion of results, a change in transcription of a co-factor gene is assumed to be limiting for feedback on the TF that regulates its expression. Because of the redundancy in the regulation of genes by PIFs, we will treat the transcriptional results for pifq mutants as being relevant for regulation by PIF4. Similarly, because of the redundancy in the regulation of genes by MYCs results for the triple mutant myc2/3/4 (myct) are treated as being relevant for regulation by MYC2.
Transcriptional Feedback on and by PIF4
Of the 34 different TFs with validated binding to the promoter of PIF4 (Fig. 2), the literature search identified 13 positive acting TFs and eight negative acting TFs on PIF4 expression (Fig. 3). Although PIF4 and other PIFs bind to the PIF4 promoter, the role of PIF4 (and other PIFs) in the transcription of the PIF4 gene has not been studied extensively. Ectopic overexpression of PIF4 results in suppression of the endogenous PIF4 gene (Shapulatov 2019). Moreover, PIF4 expression is also upregulated in a pif3 mutant compared to WT, but not in a pif1 or pif5 single mutant (Zhang et al. 2013). Overall, this suggests that PIF4 and PIF3 may be negative regulators of PIF4 transcription. Some of the negative TFs acting on PIF4 transcription are part of the clock: components of the evening complex (LUX, ELF3, ELF4: (Nusinow et al. 2011, Mizuno et al. 2014, Nieto et al. 2015, Raschke et al. 2015, Ezer et al. 2017)), clock genes PRR9 (Zhu et al. 2016; Li, Zhang et al. 2020a, b, c), PRR7 (Mizuno et al. 2014), PRR5 (Zhu et al. 2016), and the clock gene GI (Zhu et al. 2016). In contrast, clock genes LHY and CCA1 have a positive effect on PIF4 transcription (Sun et al. 2019). The transcription feedback by PIF4 on some of the clock components (Fig. 3, feedback 3 and 4) suggests an intricated balance between growth and circadian timekeeping that may result in finetuned gating of PIF4 activity over the day. Gating of PIF4 activity by the clock has also been attributed to the interaction between TOC1 and PIF4 (Soy et al. 2016; Zhu et al. 2016), but transcription of TOC1 is not under direct feedback regulation by PIF4.
Potential feedback interactions between TFs and co-factors acting on PIF4. PIF4 gene is indicated by blue line (promoter) and blue rectangle (coding sequence). PIF4 protein is indicated by blue oval. TFs with positive/negative action on transcription of the PIF4 gene are indicated in green/red blocks. TFs in black are not under transcriptional feedback by PIF4. Solid red/green arrows: negative/positive transcriptional feedback by PIF4. Dashed red lines: negative feedback on transcriptional activity of PIF4 protein. Dashed green arrow: indirect positive feedback on PIF4 transcription by PIF4 through upregulation BR synthesis and signaling which activates BZR1 (positive feedback 1). COG1 is upregulated by PIFs in a bzr1-1D mutant background (Oh et al. 2012) and interaction is assumed to also take place in a WT background (potential positive feedback 2). Negative feedback 5 indicates upregulation of co-factors repressing PIF4 protein activity (for factors between parenthesis transcriptional feedback by PIF4 is condition dependent; see Table 1). Black arrow: negative feedback of PIF4 on its own expression (negative feedback 6). Red/green numbers: negative/positive feedback on PIF4. Sources for RNA-seq data related to PIFs are in legend Table 1
The transcription factors MYB30 (Bertoni 2020; Yan et al. 2020), BZR1 (Ibanez et al. 2018) (Oh et al. 2012), a subset of TCPs (Han et al. 2019), a subset of WRKYs (Foreman et al. 2011; Sun et al. 2020), and COG1 (Wei et al. 2017) all have been identified as positive regulators of PIF4 expression. The action of BZR1, CCA1, TCPs, and WRKYs on PIF4 transcription becomes limiting during thermomorphogenesis. The extensive redundancy in TFs regulating PIF4 transcription may hide the contribution of these TFs when the expression of PIF4 is low at normal temperature.
The stimulation by PIF4 of BR biosynthesis gene expression during thermomorphogenesis and the resulting activation of BZR1 forms a positive feedback loop on PIF4 transcription by BZR1, which has previously been recognized (Ibanez et al. 2018) (Fig. 3: positive feedback 1). Our inventory identifies additional potential positive feedback on PIF4 through the positive regulator of PIF4, COG1 (Fig. 3, positive feedback 2) and through downregulation by PIF4 of the repressors of PIF4: SPCH, PRR7, and PRR5 (Lau et al. 2018) (Fig. 3, positive feedback 3). It has previously been recognized that positive feedback on PIF4 transcription poses a potential danger for out-of-control upregulation of PIF4 transcription (Ibanez et al. 2018). However, our inventory identifies multiple negative feedback interactions on PIF4 transcription or PIF4 transcriptional activity that may balance the positive feedback loops: PIF4 represses transcription of the positive TFs TCP13, LHY, WRKY26, WRKY33, and PIF7 (Fig. 3: negative feedback 4). In addition to these multiple potential negative feedback on transcription of the PIF4 gene, there is also multiple negative feedback on the activity of PIF4 protein, as PIF4 upregulates six TFs that interfere with PIF4 transcriptional activity (Fig. 3: negative feedback 5). Finally, the PIF4 protein itself may play an important role in limiting the upregulation of PIF4 transcription as PIF4 is a negative regulator of its own expression (Fig. 3: negative feedback 6).
Because feedback on the photoreceptors which affect the stability of PIF4 protein is both through PIF4 and BZR1 this is discussed separately below (Fig. 8).
Transcriptional Feedback on and by BZR1
For the 36 different TFs with validated binding to the promoter of BZR1 (Fig. 2), evidence for actual transcription regulation of BZR1 is not available. The binding of PIF1, PIF4, PIF5, and HY5 to the promoter of BZR1 does not result in altered transcription of BZR1 in PIF or HY5 mutants (Table 2). BZR1 can bind to its own promoter (Fig. 2) and BZR1 gene transcription is upregulated by the application of BR, which activates BZR1, suggesting that transcription of BZR1 is under positive auto-feedback regulation (Fig. 4; positive feedback 1). However, transcription of BZR1 is not upregulated in the bzr1-1D mutant background, suggesting that BZR1 alone is not sufficient for auto-feedback on BZR1 transcription. In total six positive feedback loops on BZR1 are identified, involving 13 co-factor genes (Fig. 4). BZR1 upregulates transcription of TF WRKY36, which enhances BZR1 target gene expression (Chen et al. 2017). Since BZR1 may target its own expression the upregulation of WRKY36 forms potential positive feedback on BZR1 (Fig. 4, positive feedback 2). The negative regulators of BZR1 protein HAT1, RD26, MYBL2, TINY, and HDA15 all are downregulated by BZR1, thus forming additional potential positive feedback on BZR1 (Fig. 4; positive feedback 3). The transcriptional feedback on BZR1-related co-factor genes was investigated in dark grown seedlings treated with BR, which activates BZR1 and BES1 (Sun et al. 2010) and in dark grown bzr1-1D mutant seedlings treated with Brassinazole (BRZ) to inhibit endogenous BR biosynthesis. The former experiment reveals targets of both BZR1 and BES1. The latter may reveal the direct targets of only BZR1 (in the form of constitutive active bzr1-1D), since activation of BES1 is limited through inhibition of endogenous BR biosynthesis by BRZ (Oh et al. 2012). The brassinosteroid-insensitive 2 (BIN2) is a kinase and a negative regulator of BZR1 protein stability and nuclear transport. The activity of BIN2 is inhibited by the activity of phosphatase BSU1 (Kim et al. 2009, 2011) and transcription of BSU1 is stimulated by BZR1, which thus forms potential positive feedback on BZR1 activity (Fig. 4, positive feedback 6). The potential reduction in BIN2 activity through upregulation of BSU1 may also lead to reduced stability of the BES1/BZR1 corepressors HAT1, RD26, TINY, and MYBL2 as all these co-repressors are substrates of BIN2 (Ye et al. 2012; Zhang et al. 2014; Jiang et al. 2019; Xie et al. 2019). While phosphorylation of BES1/BZR1 by BIN2 leads to protein degradation, BIN2 phosphorylation of HAT1, RD26, TINY, and MYBL2 stabilizes these corepressors of BZR1 activity. Therefore, reduced BIN2 activity could reduce the suppressing effect of HAT1, RD26, TINY, and MYBL2 on BZR1, resulting in an indirect positive feedback on BZR1 (Fig. 4; positive feedback through a). BZR1 is also involved in direct downregulation of HAT1, RD26, TINY, and MYBL2 (Table 2), resulting in another potential positive feedback on BZR1 (Fig. 4, positive feedback 3). BOP1 (and by analogy BOP2) inhibits the transport of BZR1 from the cytosol to the nucleus and thus negatively regulates BZR1 activity. The downregulation of BOP1 and BOP2 by BZR1 thus forms additional positive feedback on BZR1 (Fig. 4, positive feedback 4). Active BRs are conjugated by ATAF2, and upregulation of ATAF2 by BZR1 thus forms another potential positive feedback on BZR1 (Fig. 4, positive feedback 5). Thus, the negative feedback on BZR1 through BR biosynthesis and direct action of BIN2 on BZR1 (Fig. 4, negative feedback 7) is balanced by multiple potential positive feedback loops on BZR1 (Fig. 4, positive feedback 1–6,8 and a). It has already been described that transcription of some key genes in BR biosynthesis is under negative control by BES1 (Kim et al. 2009) and transcriptome analysis of the bzr1-1D mutant indicates a similar role for BZR1 in the suppression of BR biosynthesis genes (Table 2). The transcriptome of the bzr1-1D mutant reveals that also several genes encoding components of the BR receptor complex are also under negative transcriptional feedback by BZR1 (Table 2). Because feedback on the photoreceptors which affect the stability of BZR1 protein (Fig. 4 positive feedback 8) is both through BZR1 and PIF4 this is discussed separately below (see Fig. 8).
Potential feedback interactions between TFs and co-factors acting on BZR1. BZR1 gene is indicated by a blue line (promoter) and blue rectangle (coding sequence). BZR1 protein is indicated by blue oval. In the presence of BR signaling, BZR1 may show positive feedback on its own transcription through feedback loops 1, 2, 3, 4, 5, 6, and 8. These potential feedforward loops are balanced by negative feedback through 7, which results in suppression of BR biosynthesis and signaling and downregulation of BOP1/2 which targets BZR1. Solid red/green arrows: negative/positive transcriptional feedback by BZR1. Dashed red lines: indirect negative feedback on BZR1 protein. Dashed green arrow: indirect positive feedback on BZR1 protein. Red/green numbers: negative/positive feedback loops on BZR1. Sources for RNA-seq data related to BZR1 are in legend Table 2
Transcriptional Feedback on and by HY5
From the 26 different TFs with validated binding to the promoter of HY5 (Fig. 2), our literature search identifies 20 transcription factors for which either positive or negative transcription regulation of the HY5 gene has been demonstrated. However, most of these validated TFs acting on HY5 are not under transcriptional feedback by HY5 (Fig. 5, indicated in black; see Table S4). The binding of PIF4 to the promoter of HY5 does not correlate to the transcriptional regulation of HY5 by PIF4 or PIFs (Table 3). A literature search identifies nine positive-acting TFs and nine negative-acting TFs for HY5 transcription, while the action of TF MYC2 on HY5 transcription depends on light color (Ortigosa et al. 2020). The PlantPan3.0 software does not predict a HY5 binding site in the promoter of HY5 and ChIP-seq data show HY5 binding to the promoter of HYH (a close homolog of HY5), but no binding to the promoter of HY5 itself (Lee et al. 2007). However, binding of HY5 (and HYH) to T/G box in the promoter of HY5 was detected by Binkert, using dedicated ChIP qPCR analysis rather than ChIP-seq, and a potential positive auto-feedback regulation of HY5 transcription by HY5 was demonstrated (Binkert et al. 2014) (Fig. 5: positive feedback 1). In addition, there is potential positive feedback through upregulation of positive acting TFs HYH and BBX11 by HY5, especially under UVB light (Binkert et al. 2014; Yang et al. 2018; Chakraborty et al. 2019) (Fig. 5; positive feedback 2). BBX32 protein suppresses the activity of positive action of TF BBX21 on HY5 (Holtan et al. 2011). Therefore, suppression of BBX32 transcription by HY5 constitutes another potential indirect positive feedback regulation on HY5 activity (Fig. 5; positive feedback 4). Transcriptional regulation of HY5 by MYC2 is complicated as MYC2 stimulates HY5 expression under Red (R) light (Ortigosa et al. 2020), but represses HY5 transcription under Blue (B) light (Chakraborty et al. 2019) (Fig. 5; feedback 3). Under B light, the negative feedback on MYC2 thus results in potential positive feedback on HY5, while under R light the negative feedback on MYC2 results in potential negative feedback on HY5. In addition, under B light COP1 enhances the stabilization of HYH (Gangappa and Botto 2016), resulting in potential positive feedback on HY5 transcription by HYH (Fig. 5, positive feedback a). Under white light, the actual feedback may depend on the relative contribution of R and B components in the white light. The different positive feedback on HY5 is compensated by negative feedback through upregulation of TF WRKY36, which blocks HY5 transcriptional activity, and upregulation of COP1, which targets HY5 protein for degradation (Fig. 5; negative feedback 5). The upregulation of RUP2 by HY5 was only uncovered by dedicated qPCR experiments under UV light conditions (Binkert et al. 2014). RUP2, together with RUP1 simulates URV8 dimer formation, ending signaling of the UVR8 monomer in response to UV-B light. UVR8 monomer interacts with and represses WRKY36, a negative transcriptional regulator of HY5 and the UVR8 monomer disrupts COP1 function for degradation of HY5. Thus, UV light initially may result in enhanced transcription and protein stability of HY5. RUP1 and RUP2 are two proteins that promote UVR8 dimer formation and are involved in the termination of UVR8 monomer signaling. The transcriptional upregulation of both COP1 and RUP2 by HY5 (Huang et al. 2012), potentially terminates and limits UVR8 monomer activity on WRKY49 and COP1 (Binkert et al. 2014) (Fig. 5; negative feedback 6).
Potential feedback interactions between TFs and co-factors acting on HY5. HY5 gene is indicated by blue line (promoter) and blue rectangle (coding sequence). HY5 protein is indicated by blue oval. TFs with positive action on HY5 are indicated in green blocks, and TFs with negative action on HY5 are indicated in red blocks. TFs in black letters are not under transcriptional feedback by HY5. Solid red/green arrows: negative/positive transcriptional feedback by HY5. Dashed red lines: indirect negative feedback on HY5 protein. Dashed green arrow a: indirect positive feedback on HY5 under B light. Red/green numbers: negative/positive feedback loops on HY5. R: red light, B: blue light. The feedback through RUP1 and UVR8 is relevant under UV light. Sources for RNA-seq data related to HY5 are in legend Table 3
Transcriptional Feedback on and by MYC2
For most of the different TFs with validated binding to the promoter of MYC2 (Fig. 2) evidence for actual transcription regulation of MYC2 is not available. The binding of PIF4 to the promoter of PIF4 does not correlate to transcriptional regulation of MYC2 by PIF4 or PIFs (Table 4). It has been shown that binding MYC2 to its own promoter does indeed result in a direct positive auto-feedback regulation by MYC2 during short-term JA signaling (Van Moerkercke et al. 2019; Wang et al. 2019). However, under prolonged JA signaling MYC2 exerts negative feedback on its own transcription (Wang et al. 2019). In addition, HY5 has been identified as a negative regulator of MYC2 transcription in combination with an unknown factor that facilitates (indirect) binding of HY5 to the MYC2 promoter (Chakraborty et al. 2019) (Fig. 6. Feedback 1). MYC2 upregulates the expression of MYC4, which assists MYC2 in targeting downstream genes (Table 4; Fig. 6, positive feedback 2). In addition, there is positive feedback through JA/JAile signaling by transcriptional upregulation of JA biosynthesis genes by MYCs, and upregulation of GSTU20 which stimulates the conversion of JA to the active signaling component JAile. The potential higher JA and JAile levels may increase JA signaling which targets JAZ proteins (transcriptional inhibitors of MYC2 activity) for destruction (Fig. 6, positive feedback 3). The feedback on JA signaling enhances MYC2 activity, as activation of MYC2 by JA-ile signaling leads to the destruction of repressor JAZ proteins that block MYC2 activity. These positive feedback loops on MYC2 are compensated by two negative feedbacks on MYC2: MYC2 activity results in the upregulation of several JAZ genes, potentially enhancing the suppression of MYC2 activity (Fig. 6, negative feedback 4) (Chini et al. 2007). Also, HY5 is upregulated by MYC2, while HY5 together with an unidentified factor downregulates MYC2 transcription (Chakraborty et al. 2019) (Fig. 6; negative feedback 5). BPM1 is part of a protein complex that targets MYC2 for degradation and JA signaling stabilizes BPM1 (Chico et al. 2020). Therefore, the positive feedback on JA synthesis and subsequent signaling can result in both a positive effect on MYC2 (through the removal of JAZ proteins) and negative effect on MYC2 (through stabilization of BPM1) (Fig. 6, positive feedback 3, negative feedback 6). The inventory of RNA-seq data shows that transcriptional feedback on MYC2 co-factor genes seems to be more extensive in rosette plants than in seedlings (Table 4). Moreover, feedback on GSTU20 by MYC2/MYCs was the opposite in seedlings and rosette plants (Table 4).
Potential feedback interactions between TFs and co-factors acting on MYC2. MYC2 gene is indicated by blue line (promoter) and blue rectangle (coding sequence). MYC2 protein is indicated by blue oval. TFs with positive/negative action on MYC2 are indicated in green/red. TF X works together with HY5 on the transcription of MYC2. Solid red/green arrows: negative/positive transcriptional feedback by MYC2. Dashed red lines: negative interaction on MYC2 protein. Dashed green arrow a: positive effect on JA by co-factor protein action. Early: positive feedback by MYC2 on its transcription in early response, late: negative feedback by MYC2 on its own expression in late response to JA. Red/green numbers: negative/positive feedback loops on HY5. (GSTU20): transcriptional feedback by MYC2 is different in seedlings and rosette plants (Table 4). (JAZ1,3,5,6,12): these JAZ genes are under negative transcriptional control in 35S-MYC2 overexpression lines (Table 4). Sources for RNA-seq data related to MYCs are in legend Table 4
Potential for Cross-Regulation of Co-factor Genes by PIF4, BZR1, HY5, and MYC2
Besides feedback of each TF on its own co-factor genes (Fig. 1A pathway b), each of the four TFs may also interact through regulation of each other’s co-factor genes (Fig. 1A, pathway c). Figure 7A shows which BZR1-, HY5- and MYC-related co-factor genes are upregulated or downregulated by PIFs/PIF4. Results indicate the potential for interaction between the PIF4 activity through indirect feedback on the other TFs. However, there is no preferred positive or negative feedback by PIF4 on either BZR1, HY5, or MYC2 transcription/activity through transcriptional regulation of their respective co-factor genes by PIF4 (Fig. 7A). Similarly, BZR1 regulates a substantial number of PIF4-related and MYC2-related co-factor genes. However, regulation of these genes by BZR1 also does not show a preference for positive or negative feedback on either PIF4, HY5, or MYC2 (Fig. 7B). Interestingly, PIF4 upregulates DELLA genes RGL1 and RGL3, while BZR1 upregulates RGA2, but downregulates DELLA genes RGL1 and RGL2 (Fig. 7A). DELLA proteins can sequester PIF4 and JAZ proteins. The feedback by DELLAs through transcriptional by PIF4 and BZR1 on growth and defense is therefore complex. HY5 is involved in the transcription of a set of negative co-factors for PIF4 and BZR1, but with no preference for positive or negative feedback on PIF4 or BZR1, respectively (Fig. 7C). As described before, MYC2 induces the transcription of HY5, while HY5 downregulates transcription of MYC2 (Chakraborty et al. 2019, Li et al. 2020). Combined this would balance the transcription of both HY5 and MYC2, but this requires activation of MYC2 through JA-ile signaling. Indeed, this is realized through the mutual feedback on JA biosynthesis by both HY5 and MYC2 (Fig. 7C). Previously, HY5 was recognized to be involved in the expression of LOX3 (Wasternack and Hause 2013), but RNA-seq data shows that also LOX2 is regulated by HY5 (Fig. 7C). Moreover, HY5 is a positive and PIF4 is a negative regulator of PYL6, a modulator of MYC2 transcriptional activity on JAZ6 and JAZ8 (Aleman et al. 2016; Li, Shi et al. 2020a, b, c)). Combined these results could indicate reduced sensitivity to ABA-induced PYL6/MYC2 heterodimer activity in the dark (when PIF4 is active) and increased ABA sensitivity to ABA-induced PYL6/MYC2 heterodimer activity in the light (when HY5 is active) (Fig. 7C). The biological meaning of modulating MYC2 activity through differential regulation of PYL6 by PIF4 and HY5 needs further study. MYCs (MYC2) enhance the expression of one positive acting co-factor gene for PIF4 (PRE1) and two positive acting co-factor genes for BZR1 (PP2A and WRKY46) (Fig. 7D). This reveals new levels of growth and defense interaction previously not recognized: while activation of MYC2 suppresses elongation through HY5, it also increases the potential for PIF4 and BZR1 activity through upregulation of PRE1, PP2A, and WRKY46 (Fig. 7D). It could be that such activity functions as priming the plant for regaining growth when defense signaling is over (Fig. 7D).
Cross regulation of PIF4, BZR1, HY5, and MYC2 co-factor genes. A Cross-regulation of BZR1, HY5, and MYC2 co-factor genes by PIF4. B Cross-regulation of PIF4, HY5, and MYC2 co-factor genes by BZR1. C Cross-regulation of PIF4, BZR1, and MYC2 co-factor genes by HY5. D Cross regulation of PIF4, BZR1, and HY5 co-factor genes by MYC2. Positive acting co-factors are shown in green, negative acting co-factors are in red. Green arrow: transcriptional upregulation of co-factor gene by key TF. Red arrow: transcriptional downregulation by key TF
Integration of Feedback on Light and Hormone Signaling by PIF4, BZR1, HY5, and MYC2
Of the five co-factor genes shared by all four TFs (HY5, PHYB, CRY1, CRY2, COP1; Fig. 2B), PHYB is co-regulated by PIF4 and BZR1, COP1 is co-regulated by BZR1 and HY5, HY5 is coregulated by PIFs and HY5, while CRY1 is only regulated by BZR1 and CRY2 only by PIF4 (Tables 1, 2, 3, and 4). Combined, this demonstrates how transcriptional regulation of growth (by PIF4, BZR1, HY5) and defense (by MYC2) potentially is linked through feedback on shared co-factor genes. This reveals multiple levels that effectuate the trade-off between growth and defense, many of which have not been recognized before.
PIF4 protein is stabilized by nuclear COP1 activity, while the nuclear complex SCFCOP1/SPA1 targets HY5 for protein degradation. Nuclear COP1 is destabilized by activated PHY, CRY or UVR8 photoreceptors, respectively (Huq and Quail 2002; Lorrain et al. 2008; Hayes et al. 2017; Tavridou et al. 2020), so in general, growth is stimulated in the dark (stable PIF4) and suppressed in the light (stable HY5). BZR1 protein is by default targeted for degradation after phosphorylation by the kinase BIN2 (He et al. 2002) and BZR1 protein is stabilized when the activity of BIN2 is inhibited under BR signaling (Sellaro et al. 2009). BZR1 protein may also be targeted for degradation in response to specific phytohormones, such as through interaction with the SCFMAX2/D14 strigolactone receptor complex (Wang et al. 2013a, b, Li et al. 2017). MYC proteins are targeted by the CUL3BPM E3 ubiquitin ligases, while the stability of BPM protein is enhanced by Jasmonic Acid signaling. Thus, JA-signaling alleviates repression of MYC2 activity but limits MYC2 activity in a negative feedback loop that regulates MYC protein stability (Jung et al. 2015; Chico et al. 2020). A summary of our inventory shows that PIF4 and BZR1 act antagonistically at multiple levels to balance positive and negative feedback on growth (Fig. 8). BR biosynthesis gene expression is upregulated by PIF4 but downregulated by BZR1. The upregulation of BR synthesis by PIF4 and potential subsequent BR signaling results in positive feedback on growth through BZR1 activated PIF4 expression during thermomorphogenesis (Ibanez et al. 2018) (Fig. 8). Such positive feedback is potentially dangerous, as it may result in uncontrolled upregulation of PIF4 by BZR1 (Ibanez et al. 2018). However, PIF4 transcription is restrained through transcriptional feedback through numerous negative acting co-factor genes. While the contribution of each single co-factor to negative feedback on PIF4 may be small, the combined feedback may help control PIF4 expression and activity, especially during heat stress.
Integrated feedback in growth by PIF4, BZR1, and HY5 and balancing growth with defense by HY5 and MYC2. Green/red arrows: positive/negative transcriptional feedback. Solid ovals: dual regulation by PIFs and BZR1. Dashed rectangles: dual regulation by HY5 and MYC2. The figure illustrates potential feedback in growth through dual feedback on BR synthesis (PIFs and BZR1), JA (HY5 and.MYCs), photoreceptors PHYB (PIFs and BZR1), UVR8 (PIFs and BZR1) and triple feedback on COP1 (PIFs, BZR1, HY5) and the balance between growth and defense through potential dual feedback on HY5 (HY5 and MYCs), MYC2 (HY5 and MYCs) and JA biosynthesis (HY5 and MYCs)
The positive feedback through BR synthesis and signaling on PIF4 is balanced by the negative regulation of BR biosynthesis and BR signaling genes by BZR1. In addition, the expression of PHYB and UVR8 is upregulated by PIF4 (Tables 1–2), which potentially increases negative feedback on growth: Light-activated PHYB directly interacts with PIF4 protein, resulting in increased mutual turnover (Huq and Quail 2002; Ni et al. 2014; Ma et al. 2016; Pedmale et al. 2016). PHYB and UVR8, when light activated, also reduce SPA1/COP1 activity. SPA1 phosphorylates PIF4 in vitro and SPA1 activity is required for the stabilization of PIF4, especially during thermomorphogenesis (Lee et al. 2020). Reduced SPA1/COP1 activity therefore results in negative feedback on PIF4, but positive feedback on HY5, both resulting in suppression of growth (Huq and Quail 2002; Lorrain et al. 2008; Hayes et al. 2017; Tavridou et al. 2020). The negative feedback on growth through PHYB and UVR8 by PIF4 is balanced by the activity of BZR1, which upregulates the expression of PHYB and UVR8 (Fig. 8). Regulation of expression of COP1 provides negative feedback growth as PIF4 and BZR1 both downregulate the expression of COP1. Under UV light HY5 can also induce transcription of COP1. Potentially this could result in negative feedback on HY5 activity and positive feedback on PIF4 activity. However, under UV the HY5 protein COP1/SPA interaction is interrupted by UVR8. No contribution of HY5 to COP1 expression is detected under other light conditions, but if a single contribution of HY5 is low, COP1 will not be detected as a differentially expressed gene.
The dual regulation by PIF4 and BZR1 has been described before BR biosynthesis genes and relates to different functions of BZR1 homodimers and PIF4/BZR1 heterodimer, respectively (Oh et al. 2012). Supposedly, BES1 and BZR1 monomers act as repressors when they bind to the promoter of BR biosynthesis genes, especially during the day when PIF4 protein levels are low (Sun et al. 2010; Wang et al. 2012). However, at night, when PIF4 protein levels are high, PIF4 binds to BES1/BZR1 to form a heterodimer which activates transcription of BR biosynthesis genes (Sun et al. 2010; Oh et al. 2012). Further studies are needed to determine whether a similar mechanism operates for diurnal PIF4/BZR1 mediated transcriptional regulation of the photoreceptor genes PHYB and UVR8. The synergistic interaction between BZR1 and PIF4 also operates downstream of PIF4 and BZR1, as thousands of common downstream target genes are synergistically co-regulated by PIF4 and BZR1 (Oh et al. 2012). SPA1 is also under dual regulation by PIF4 and BZR1, but here PIF4 represses, while BZR1 stimulates transcription SPA1.
Most central to balance activity of PIF4 may be the regulation by PIF4 itself. BZR1 upregulates the expression of PIF4. BZR1 and PIF4 were shown to synergistically stimulate the expression of thousands of common target genes, but the set of common target genes does not include PIF4 (Oh et al. 2012). However, because of the extensive redundant regulation of PIF4 transcription and activity, such dual regulation by PIF4/BZR1 may go unnoticed in single mutant studies. When expressed at high levels, ectopic PIF4 downregulates endogenous PIF4 expression, so PIF4 itself may be the most crucial factor to restrain uncontrolled PIF4 expression (Shapulatov 2019). Further studies are needed to determine the role of specific PIF4/BZR1 homodimers and heterodimers in the regulation of expression of SPA1 and PIF4. Of the blue light receptors, only CRY2 is under positive transcriptional feedback by PIFs, and under this condition, CRY2 may limit PIF4 activity. CRY2 protein is light-labile rapidly down-regulated by blue light as a function of light intensity (Lin et al. 1998). The positive transcriptional regulation of CRY2 by the light labile PIF proteins, and negative feedback of activated CRY proteins on PIF4, may therefore be most relevant under low light intensities. At higher B light intensities, the negative feedback on PIF4 through CRY1 becomes more important, and this is stimulated by BZR1 (Fig. 8).
Growth and defense are mostly balanced through mutual transcriptional regulation of HY5 and MYC2, by HY5 and MYC2 as previously described (Ortigosa et al. 2020). Positive feedback in defense is through upregulation of JA biosynthesis genes and signaling genes by MYC2, but also upregulation of some JA biosynthesis genes by HY5 (Figs. 7 and 8). In addition, defense and growth are linked through transcriptional regulation of PIF4 and BZR1 related positive acting co-factor genes by MYC2, resulting in potential positive feedback on growth by MYCs (Fig. 7). This may partly compensate for the negative effect on growth by MYC2 through HY5.
Recently the temporal transcriptional response to W, B, R, or FR light was determined for Arabidopsis seedlings (Kurihara et al. 2020). Because light plays an important role in the regulation of PIF4 and HY5 protein stability, the role of light quality on potential feedback through co-factor genes on PIF4 or HY5, respectively, was determined. Of the 102 co-factors identified for PIF4 (Table S2), 58 genes are under transcriptional feedback by PIF4 (Table 1). PIF4 protein is unstable in light, and therefore, it may be expected that transcription these co-factor genes for PIF4 is affected by light (Table S10). Indeed, 39 co-factor genes show light regulation (Table S10) and 27 of these co-factor genes show both regulations by PIF4 and by light (Table S10, grey cells). As expected, many co-factor genes seem to be under reciprocal control by PIFs and light. For instance, HSL1 is upregulated by PIFs (Table 1) but downregulated by light conditions that result in the destabilization of PIFs (Table S10A). In contrast, negative co-factor genes PRR9, HYH, PIL1, HFR1, PAR1, PAR2, HEC2, AHL27/29, and RGA1/2 are downregulated by PIFs (Table 1), but upregulated by light (Table S10B), indicating that PIFs may act as a suppressor for these genes in the dark and that other TFs beside PIF4 are responsible for the upregulation in the light. Overall, in the light regulation of PIF4-related co-factor genes, there is no consistent positive or negative feedback on PIF4 (positive feedback indicated by green cells, negative feedback indicated by red cells in Table S7-S8).
Of the 57 co-factor genes identified for HY5 (Table S3), 13 co-factor genes are under some form of light-dependent transcriptional control (Table S11). Twelve of these 13 co-factor genes are also under direct transcriptional control of HY5 (Table S11, grey cells). Transcriptional light regulation of HY5 co-factor genes results mostly in potential negative feedback on HY5, both by suppression of all positive acting, light-regulated, co-factor genes and by upregulation of most negative acting, light-regulated, co-factor genes (Table S11). Because HY5 protein is stabilized in the light, this suggests that HY5 acts as a suppressor for the positive acting co-factor genes and as an activator for the negative acting co-factor genes. Transcription of HY5 and HYH is upregulated by HY5 and light, and this positive feedback on HY5 is balanced by the transcriptional upregulation of negative acting co-factors COP1 and RUP2. In addition, ten HY5 co-factor genes under that are under transcriptional feedback by HY5 (Table 3) are not light-regulated.
Comparing PIF-, BZR1-, HY5-, and MYC-Related ChIP-seq and RNA-seq Data
Although DNA ChIP-seq data indicate a central role for PIF4 by binding to the promoters of PIF4, BZR1, HY5, and MYC2 (Fig. 2), only the PIF4 gene itself is under redundant transcriptional control by PIFs (Table 1). Of the combined set of 213 co-factor genes that affect the activity of PIF4, BZR1, HY5, and/or MYC2 protein activity, 72 co-factor genes are positive targets in the ChIP-seq experiments. Of these 72 co-factor genes, only 22 genes are under transcriptional control by one or more of these TFs. In contrast, 43 co-factor genes are transcriptionally affected by either PIF4, BZR1, HY5, and/or MYC2, without being the target of these TFs in the DNA ChIP-seq experiments. This may be in part due to detection limitations in DNA ChIP-seq experiments. For instance, the binding of HY5 to the HY5 and MYC2 promoter was only detected using the more dedicated ChIP-PCR experiments (Binkert et al. 2014; Chakraborty et al. 2019). Alternatively, transcription of these co-factor genes may be indirectly regulated by either PIF4, BZR1, HY5, and/or MYC2. Some genes bound by one or more PIF proteins do not show altered expression in the pif4 or pif-q mutant, indicating that the PIF binding does not influence the expression of these genes. Indeed, most PIF4-related co-factor genes that show altered expression in single or multiple PIF mutant backgrounds are not on the list of targets of PIF protein binding (Table 1). This either indicates that these transcriptional feedback interactions are indirect, or it indicates that DNA ChIP data are incomplete, or that conditions of DNA ChIP data differed from conditions used for transcriptome profiling (Tables 1, 2, 3, and 4).
The PIF4-, BZR1-, HY5-, and MYC2-related ChIP-seq and RNA-seq data sets used in this study were compared using cluster analysis (Fig. 9). The ChIP-seq data sets are all part of cluster B in Fig. 9, but do no cluster with most RNA-seq data sets, indicating that binding of TF to gene only shows low correlation to differential expression of that gene. The cluster analysis indicates three major clusters, each with some sub-cluster pairs. Table S6 shows the GO-enrichment analysis of the gene sets in the three major clusters. Only the gene set targeted by MYC2 clusters closely to the genes upregulated by MYC2 (Fig. 9, cluster B3). Moreover, the gene set targeted by HY5 closely clusters with the gene set targeted by MYC2, indicating the potential for interaction with the same genes between HY5 and MYC2 (Fig. 9, cluster B3). Most remarkably, the set of genes upregulated by MYC2 in 28-day-old plants clusters closely to genes downregulated by MYC2 in 15-day-old seedlings, indicating that the regulation of genes may change over time (Fig. 9, cluster A3). The GO-enrichment analysis of this cluster indicates that this mainly concerns genes involved in glucosinolate biosynthesis (Table S6). The gene sets in cluster A1 confirm the opposite regulation of PIFs/BZR1 and HY5 in growth (Fig. 9, cluster A1). Remarkably, both the PIF downregulated and BZR1 downregulated gene sets cluster closely to the BZR1 target gene set, suggesting that PIFs may play an additional role in the repressor function of BZR1 at target genes (Fig. 9, cluster B1). However, the GO-enrichment analysis does not indicate that this involves genes with a specified biological function as it mainly involves unclassified genes (Table S6). The gene set up-regulated by MYCs closely clusters with the set of genes down-regulated by PIF4 (Fig. 9, cluster C2). This mainly involves genes with function in ethylene signaling, hypoxia, and wounding (Table S6).
Cluster analysis of ChIP-seq and RNA-seq data sets used in this study. Three main clusters (A, B, C) and sub-clusters are indicated. Gray: ChIP-seq data, Red: RNA-seq data: downregulated by TF or in genotype indicated, Green: RNA-seq data: upregulated by TF or in genotype indicated. For references see legends of Tables 1, 2, 3, and 4
Gene sets downregulated by BZR1 in WT background and those down-regulated by bzr1-1D in pifq mutant background grown on BRZ do not cluster together (Fig. 9). This indicates that in a background where BR biosynthesis is blocked, BZR1 repressed target genes may require the additional action of PIFs (Sun et al. 2010). In contrast, the gene sets upregulated by BZR1 cluster closely with genes upregulated by bzr1-1D in the pifq mutant background, indicating that the positive action of BZR1 may not be dependent on PIFs (Fig. 9, cluster C1) where BZR1 by bzr1-1D require the co-action of PIFs (Sun et al. 2010).
Concluding Remarks
Each of the four selected TFs is under both feed forward and feed backward regulation by co-factors, and for PIF4 and BZR1 feedback through co-factors is most redundant. For PIF4, BZR1, and MYC2 the feedback through transcriptional regulation of their co-factor genes is mostly negative (downregulation of positive acting factors, upregulation of negative acting factors), while for HY5 the identified feedback is mostly positive (upregulation of positive acting factors and downregulation of negative acting factors) (Fig. 10). A feedforward regulation results in a prolonged activity of the TF(s), while the feed-feedback regulation may be needed to terminate the activity of the TFs (Lee et al. 2007). The extensive redundancy in negative feedback in the transcriptional regulation of growth strongly buffers against the effect of a single (a) biotic, mutant, or chemical perturbation. The implications of how growth and defense are linked by feedback transcription through co-factor genes should therefore be studied by probing or modeling combinatorial perturbations. The transcriptional regulation of PIF4, BZR1, HY5, and MYC2 is complicated by the fact that the action of some TFs (e.g., PIF4 and BZR1/BES1) at common target genes depends on homodimer or heterodimer formation. For instance, growth BZR1 and PIF4 share a sub-set of downstream genes and presumably activate transcription of these genes as heterodimers (PIF4/BZR1) but may block transcription as homodimers (He et al. 2005; Oh et al. 2012, Planas-Riverola et al. 2019), while for another subset of common target genes they activate genes as homodimer (BZR1 or BES1), but block transcription as heterodimer with PIF4 (Martinez et al. 2018). The cluster analysis shows that most BZR1 repressed genes seem to require PIF co-action, while most positive targets of BZR1 seem to be independent of PIFs (Fig. 9 cluster C1). Therefore, BZR1-PIF4 heterodimer may mainly function as a transcription repressor for a subset of target genes, while BZR1 homodimer may function as an activator for another subset of target genes. The actual feedback by each of these TFs on target genes may be a function of their relative abundance, the potential for dimer formation, context, and sequence at the promoter binding site.
Summary of transcriptional feedback on co-factor genes for PIF4, BZR1, HY5, and MYC2 (data from Tables 1, 2, 3, and 4). Solid arrows up/down from TF: co-factor genes under positive (green) or negative (red) transcriptional control by PIF4, BZR1, HY5, or MYC2. Striped arrows: positive or negative transcriptional control of co-factor gene depends on condition. The thickness of the arrow relates to the total number of co-factors under transcriptional feedback by the TF. Arrows from co-factors to TF: positive (green) or negative (red) feedback by co-factors on TF. The + or – on top indicates an overall more positive ( +) or negative (-) feedback on PIF4, BZR1, HY5, and MYC2 by their related co-factors, respectively
Major transcriptional feedback is through transcriptional control of photoreceptor co-factor genes and the SPA1/COP1 co-factor genes and integrates the action of all four TFs (Fig. 8). The actual potential for coordinate transcription of PIF4, BZR1, HY5, and MYC2 may be larger than shown here because there are 15 other TFs besides PIF4 with shared validated binding to the promoter of these TF genes (Fig. 2) and it still needs to be determined if any of these have an active role in transcription of these four TFs and/or their related co-factor genes. The identified transcriptional feedback regulation by the co-factor genes may not be universal, but limited to certain tissue (Chaiwanon and Wang 2015) or cell type-specific (Lau et al. 2018); it may be limited to certain environmental conditions such as heat (Ibanez et al. 2018) or it may depend on developmental stage as shown for the myct mutant (Table S8; (Schweizer et al. 2013; Van Moerkercke et al. 2019)).
Genome-wide comparison of Arabidopsis and several crop species indicates that the key components of light signaling (PIFs, HY5) and BR signaling (BZR1 and BES1) are conserved in crops (Wang et al. 2017; Han et al. 2019a, b; Thalmann et al. 2019). Also, defense signaling pathway components are conserved between Arabidopsis and crops, but there are also significant differences (Anderson et al. 2005). For instance, PIFs, BZR1 and MYC genes from Arabidopsis equally relate to one or more rice genes and HY5 from Arabidopsis has multiple paralogues in rice (Wang et al. 2017). The feedback regulation on PIF4 through PRRs and HFR1 may be different in monocots, as rice has multiple paralogues for PRR5, no orthologue for PRR9, multiple paralogues for PRR7, and no orthologue for HFR1 (Wang et al. 2017). The myriad of potential positive and negative feedback, especially on PIF4, indicates the large pool of options that are available in nature to obtain a balanced expression of PIFs to control growth. It is to be expected that the relative contribution of the potential positive and negative co-factors in the control of the activity of these TFs may differ in different plant species, while overall regulation of growth remains the same. Therefore, the better we understand the potential of interactions between growth and defense components, the better we may be able to interpret difference in transcriptional regulation in crop species.
References
Adams E, Diaz C, Hong J-P, Shin R (2014) 14-3-3 proteins participate in light signaling through association with PHYTOCHROME INTERACTING FACTORs. Int J Mol Sci 15(12):22801–22814
Aleman F, Yazaki J, Lee M, Takahashi Y, Kim AY, Li Z, Kinoshita T, Ecker JR, Schroeder JI (2016) An ABA-increased interaction of the PYL6 ABA receptor with MYC2 transcription factor: a putative link of ABA and JA signaling. Sci Rep 6:28941
Anderson JP, Thatcher LF, Singh KB (2005) Plant defence responses: conservation between models and crops. Funct Plant Biol 32(1):21–34
Bertoni G (2020) MYB30 regulates photomorphogenesis via Interactions with active phytochromes and PIFs. Plant Cell 32(7):2065–2066
Binkert M, Kozma-Bognar L, Terecskei K, De Veylder L, Nagy F, Ulm R (2014) UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 26(10):4200–4213
Camoni L, Visconti S, Aducci P, Marra M (2018) 14–3–3 proteins in plant hormone signaling: doing several things at once. Front Plant Sci 9(297)
Chaiwanon J, Wang ZY (2015) Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr Biol 25(8):1031–1042
Chakraborty M, Gangappa SN, Maurya JP, Sethi V, Srivastava AK, Singh A, Dutta S, Ojha M, Gupta N, Sengupta M, Ram H, Chattopadhyay S (2019) Functional interrelation of MYC2 and HY5 plays an important role in Arabidopsis seedling development. Plant J 99(6):1080–1097
Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in Brassinosteroid-regulated plant growth and drought responses. Plant Cell 29(6):1425–1439
Chico JM, Fernandez-Barbero G, Chini A, Fernandez-Calvo P, Diez-Diaz M, Solano R (2014) Repression of Jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in Arabidopsis. Plant Cell 26(5):1967–1980
Chico JM, Lechner E, Fernandez-Barbero G, Canibano E, Garcia-Casado G, Franco-Zorrilla JM, Hammann P, Zamarreno AM, Garcia-Mina JM, Rubio V, Genschik P, Solano R (2020) CUL3(BPM) E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc Natl Acad Sci U S A 117(11):6205–6215
Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448(7154):666–671
Delker C, Sonntag L, James GV, Janitza P, Ibanez C, Ziermann H, Peterson T, Denk K, Mull S, Ziegler J, Davis SJ, Schneeberger K, Quint M (2014) The DET1-COP1-HY5 pathway constitutes a multipurpose signaling module regulating plant photomorphogenesis and thermomorphogenesis. Cell Rep 9(6):1983–1989
Ezer D, Jung JH, Lan H, Biswas S, Gregoire L, Box MS, Charoensawan V, Cortijo S, Lai X, Stockle D, Zubieta C, Jaeger KE, Wigge PA (2017) The evening complex coordinates environmental and endogenous signals in Arabidopsis. Nat Plants 3:17087
Fernandez-Calvo P, Chini A, Fernandez-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, Pauwels L, Witters E, Puga MI, Paz-Ares J, Goossens A, Reymond P, De Jaeger G, Solano R (2011) The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23(2):701–715
Foreman J, Johansson H, Hornitschek P, Josse EM, Fankhauser C, Halliday KJ (2011) Light receptor action is critical for maintaining plant biomass at warm ambient temperatures. Plant J 65(3):441–452
Gampala SS, Kim TW, He JX, Tang W, Deng Z, Bai MY, Guan S, Lalonde S, Sun Y, Gendron JM, Chen H, Shibagaki N, Ferl RJ, Ehrhardt D, Chong K, Burlingame AL, Wang ZY (2007) An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell 13(2):177–189
Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9(10):1353–1365
Gangappa SN, Kumar SV (2017) DET1 and HY5 control PIF4-mediated thermosensory elongation growth through distinct mechanisms. Cell Rep 18(2):344–351
Han X, Chang X, Zhang Z, Chen H, He H, Zhong B, Deng XW (2019a) Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol Plant 12(6):847–862
Han X, Yu H, Yuan R, Yang Y, An F, Qin G (2019b) Arabidopsis transcription factor TCP5 controls plant thermomorphogenesis by Positively Regulating PIF4 Activity. iScience 15:611–622
Hayes S, Sharma A, Fraser DP, Trevisan M, Cragg-Barber CK, Tavridou E, Fankhauser C, Jenkins GI, Franklin KA (2017) UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis. Curr Biol 27(1):120–127
He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307(5715):1634–1638
He JX, Gendron JM, Yang Y, Li J, Wang ZY (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A 99(15):10185–10190
Holtan HE, Bandong S, Marion CM, Adam L, Tiwari S, Shen Y, Maloof JN, Maszle DR, Ohto MA, Preuss S, Meister R, Petracek M, Repetti PP, Reuber TL, Ratcliffe OJ, Khanna R (2011) BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol 156(4):2109–2123
Huai J, Zhang X, Li J, Ma T, Zha P, Jing Y, Lin R (2018) SEUSS and PIF4 coordinately regulate light and temperature signaling pathways to control plant growth. Mol Plant 11(7):928–942
Huang X, Ouyang X, Yang P, Lau OS, Li G, Li J, Chen H, Deng XW (2012) Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 24(11):4590–4606
Huang X, Zhang Q, Jiang Y, Yang C, Wang Q, Li L (2018) Shade-induced nuclear localization of PIF7 is regulated by phosphorylation and 14–3–3 proteins in Arabidopsis. Elife 7
Huq E, Quail PH (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21(10):2441–2450
Ibanez C, Delker C, Martinez C, Burstenbinder K, Janitza P, Lippmann R, Ludwig W, Sun H, James GV, Klecker M, Grossjohann A, Schneeberger K, Prat S, Quint M (2018) Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr Biol 28(2):303–310 e303
Jiang H, Tang B, Xie Z, Nolan T, Ye H, Song GY, Walley J, Yin Y (2019) GSK3-like kinase BIN2 phosphorylates RD26 to potentiate drought signaling in Arabidopsis. Plant J 100(5):923–937
Jung C, Zhao P, Seo JS, Mitsuda N, Deng S, Chua NH (2015) PLANT U-BOX PROTEIN10 regulates MYC2 stability in Arabidopsis. Plant Cell 27(7):2016–2031
Kim B, Jeong YJ, Corvalan C, Fujioka S, Cho S, Park T, Choe S (2014) Darkness and gulliver2/phyB mutation decrease the abundance of phosphorylated BZR1 to activate brassinosteroid signaling in Arabidopsis. Plant J 77(5):737–747
Kim TW, Guan S, Burlingame AL, Wang ZY (2011) The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell 43(4):561–571
Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang JX, Sun Y, Burlingame AL, Wang ZY (2009) Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11(10):1254–1260
Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA (2009) High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19(5):408–413
Kurihara Y, Makita Y, Shimohira H, Matsui M (2020) Time-course transcriptome study reveals mode of bZIP transcription factors on light exposure in Arabidopsis. Int J Mol Sci 21(6)
Lau OS, Song Z, Zhou Z, Davies KA, Chang J, Yang X, Wang S, Lucyshyn D, Tay IHZ, Wigge PA, Bergmann DC (2018) Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr Biol 28(8):1273–1280 e1273
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19(3):731–749
Lee S, Paik I, E. Huq E (2020) SPAs promote thermomorphogenesis by regulating the phyB-PIF4 module in Arabidopsis. Development 147(19)
Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH (2008) Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18(23):1815–1823
Leivar P, Tepperman JM, Cohn MM, Monte E, Al-Sady B, Erickson E, Quail PH (2012) Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in <em>Arabidopsis</em>. Plant Cell 24(4):1398–1419
Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, Quail PH (2009) Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21(11):3535–3553
Li C, Nozue K, Maloof JN (2020a) MYCs and PIFs act independently in Arabidopsis growth regulation. G3 (Bethesda) 10(5):1797–1807
Li C, Shi L, Wang Y, Li W, Chen B, Zhu L, Fu Y (2020b) Arabidopsis ECAP is a new adaptor protein that connects JAZ repressors with the TPR2 co-repressor to suppress Jasmonate-responsive anthocyanin accumulation. Mol Plant 13(2):246–265
Li N, Zhang Y, He Y, Wang Y, Wang L (2020c) Pseudo response regulators regulate photoperiodic hypocotyl growth by repressing PIF4/5 transcription. Plant Physiol 183(2):686–699
Li QF, He JX (2016) BZR1 interacts with HY5 to mediate brassinosteroid- and light-regulated cotyledon opening in arabidopsis in darkness. Mol Plant 9(1):113–125
Li QF, Huang LC, Wei K, Yu JW, Zhang CQ, Liu QQ (2017) Light involved regulation of BZR1 stability and phosphorylation status to coordinate plant growth in Arabidopsis. Biosci Rep 37(2)
Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR (1998) Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc Natl Acad Sci U S A 95(5):2686–2690
Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53(2):312–323
Lu XD, Zhou CM, Xu PB, Luo Q, Lian HL, Yang HQ (2015) Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol Plant 8(3):467–478
Ma D, Li X, Guo Y, Chu J, Fang S, Yan C, Noel JP, Liu H (2016) Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc Natl Acad Sci U S A 113(1):224–229
Martinez C, Espinosa-Ruiz A, de Lucas M, Bernardo-Garcia S, Franco-Zorrilla JM, Prat S (2018) PIF4-induced BR synthesis is critical to diurnal and thermomorphogenic growth. EMBO J 37(23)
Mizuno T, Nomoto Y, Oka H, Kitayama M, Takeuchi A, Tsubouchi M, Yamashino T (2014) Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana. Plant Cell Physiol 55(5):958–976
Ni W, Xu SL, Tepperman JM, Stanley DJ, Maltby DA, Gross JD, Burlingame AL, Wang ZY, Quail PH (2014) A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344(6188):1160–1164
Nieto C, Lopez-Salmeron V, Daviere JM, Prat S (2015) ELF3-PIF4 interaction regulates plant growth independently of the Evening Complex. Curr Biol 25(2):187–193
Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farre EM, Kay SA (2011) The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475(7356):398–402
Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY (2014) Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife 3
Oh E, Zhu JY, Wang ZY (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14(8):802–809
Ortigosa A, Fonseca S, Franco-Zorrilla JM, Fernandez-Calvo P, Zander M, Lewsey MG, Garcia-Casado G, Fernandez-Barbero G, Ecker JR, Solano R (2020) The JA-pathway MYC transcription factors regulate photomorphogenic responses by targeting HY5 gene expression. Plant J 102(1):138–152
Pedmale UV, Huang SC, Zander M, Cole BJ, Hetzel J, Ljung K, Reis PAB, Sridevi P, Nito K, Nery JR, Ecker JR, Chory J (2016) Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164(1–2):233–245
Pfeiffer A, Shi H, Tepperman JM, Zhang Y, Quail PH (2014) Combinatorial complexity in a transcriptionally centered signaling hub in Arabidopsis. Mol Plant 7(11):1598–1618
Planas-Riverola A, Gupta A, Betegon-Putze I, Bosch N, Ibanes M, Cano-Delgado AI (2019) Brassinosteroid signaling in plant development and adaptation to stress. Development 146(5)
Qiu Y (2020) Regulation of PIF4-mediated thermosensory growth. Plant Sci 297:110541
Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, van Zanten M (2016) Molecular and genetic control of plant thermomorphogenesis. Nat Plants 2:15190
Raschke A, Ibanez C, Ullrich KK, Anwer MU, Becker S, Glockner A, Trenner J, Denk K, Saal B, Sun X, Ni M, Davis SJ, Delker C, Quint M (2015) Natural variants of ELF3 affect thermomorphogenesis by transcriptionally modulating PIF4-dependent auxin response genes. BMC Plant Biol 15:197
Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW (2003) The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 17(21):2642–2647
Schweizer F, Fernandez-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P (2013) Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25(8):3117–3132
Sellaro R, Hoecker U, Yanovsky M, Chory J, Casal JJ (2009) Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr Biol 19(14):1216–1220
Shapulatov U (2019) Exploring the molecular hub in plant elongation responses: regulation of PHYs and PIFs, Wageningen University
Soy J, Leivar P, Gonzalez-Schain N, Martin G, Diaz C, Sentandreu M, Al-Sady B, Quail PH, Monte E (2016) Molecular convergence of clock and photosensory pathways through PIF3-TOC1 interaction and co-occupancy of target promoters. Proc Natl Acad Sci U S A 113(17):4870–4875
Srivastava M, Srivastava AK, Orosa-Puente B, Campanaro A, Zhang C, Sadanandom A (2020) SUMO conjugation to BZR1 enables brassinosteroid signaling to integrate environmental cues to shape plant growth. Curr Biol 30(8):1410–1423 e1413
Sun Q, Wang S, Xu G, Kang X, Zhang M, Ni M (2019) SHB1 and CCA1 interaction desensitizes light responses and enhances thermomorphogenesis. Nat Commun 10(1):3110
Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, Patil S, Kim TW, Ji H, Wong WH, Rhee SY, Wang ZY (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19(5):765–777
Sun Y, Liu Z, Guo J, Zhu Z, Zhou Y, Guo C, Hu Y, Li J, Shangguan Y, Li T, Hu Y, Wu R, Li W, Rochaix JD, Miao Y, Sun X (2020) WRKY33-PIF4 loop is required for the regulation of H2O2 homeostasis. Biochem Biophys Res Commun 527(4):922–928
Tavridou E, Pireyre M, Ulm R (2020) Degradation of the transcription factors PIF4 and PIF5 under UV-B promotes UVR8-mediated inhibition of hypocotyl growth in Arabidopsis. Plant J 101(3):507–517
Thalmann M, Coiro M, Meier T, Wicker T, Zeeman SC, Santelia D (2019) The evolution of functional complexity within the beta-amylase gene family in land plants. BMC Evol Biol 19(1):66
Van Moerkercke A, Duncan O, Zander M, Simura J, Broda M, Vanden Bossche R, Lewsey MG, Lama S, Singh KB, Ljung K, Ecker JR, Goossens A, Millar AH, Van Aken O (2019) A MYC2/MYC3/MYC4-dependent transcription factor network regulates water spray-responsive gene expression and jasmonate levels. Proc Natl Acad Sci U S A 116(46):23345–23356
Wang C, Shang JX, Chen QX, Oses-Prieto JA, Bai MY, Yang Y, Yuan M, Zhang YL, Mu CC, Deng Z, Wei CQ, Burlingame AL, Wang ZY, Sun Y (2013a) Identification of BZR1-interacting proteins as potential components of the brassinosteroid signaling pathway in Arabidopsis through tandem affinity purification. Mol Cell Proteomics 12(12):3653–3665
Wang H, Li S, Li Y, Xu Y, Wang Y, Zhang R, Sun W, Chen Q, Wang XJ, Li C, Zhao J (2019) MED25 connects enhancer-promoter looping and MYC2-dependent activation of jasmonate signalling. Nat Plants 5(6):616–625
Wang H, Yang C, Zhang C, Wang N, Lu D, Wang J, Zhang S, Wang ZX, Ma H, Wang X (2011) Dual role of BKI1 and 14-3-3 s in brassinosteroid signaling to link receptor with transcription factors. Dev Cell 21(5):825–834
Wang P, Hendron RW, Kelly S (2017) Transcriptional control of photosynthetic capacity: conservation and divergence from Arabidopsis to rice. New Phytol 216(1):32–45
Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X (2013b) Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev Cell 27(6):681–688
Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46:701–724
Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2(4):505–513
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111(6):1021–1058
Wei Z, Yuan T, Tarkowská D, Kim J, Nam HG, Novák O, He K, Gou X, Li J (2017) Brassinosteroid biosynthesis is modulated via a transcription factor cascade of COG1, PIF4, and PIF5. Plant Physiol 174(2):1260–1273
Xie Z, Nolan T, Jiang H, Tang B, Zhang M, Li Z, Yin Y (2019) The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 31(8):1788–1806
Yan Y, Li C, Dong X, Li H, Zhang D, Zhou Y, Jiang B, Peng J, Qin X, Cheng J, Wang X, Song P, Qi L, Zheng Y, Li B, Terzaghi W, Yang S, Guo Y, Li J (2020) MYB30 is a key negative regulator of Arabidopsis photomorphogenic development that promotes PIF4 and PIF5 protein accumulation in the light. Plant Cell 32(7):2196–2215
Yang Y, Liang T, Zhang L, Shao K, Gu X, Shang R, Shi N, Li X, Zhang P, Liu H (2018) UVR8 interacts with WRKY36 to regulate HY5 transcription and hypocotyl elongation in Arabidopsis. Nat Plants 4(2):98–107
Ye H, Li L, Guo H, Yin Y (2012) MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A 109(49):20142–20147
You Y, Zhai Q, An C, Li C (2019) LEUNIG_HOMOLOG mediates MYC2-dependent transcriptional activation in cooperation with the coactivators HAC1 and MED25. Plant Cell 31(9):2187–2205
Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P, Rodermel S, Yin Y (2011) A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 65(4):634–646
Zhang D, Ye H, Guo H, Johnson A, Zhang M, Lin H, Yin Y (2014) Transcription factor HAT1 is phosphorylated by BIN2 kinase and mediates brassinosteroid repressed gene expression in Arabidopsis. Plant J 77(1):59–70
Zhang Y, Mayba O, Pfeiffer A, Shi H, Tepperman JM, Speed TP, Quail PH (2013) A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet 9(1):e1003244
Zhao L, Peng T, Chen CY, Ji R, Gu D, Li T, Zhang D, Tu YT, Wu K, Liu X (2019) HY5 interacts with the histone deacetylase HDA15 to repress hypocotyl cell elongation in photomorphogenesis. Plant Physiol 180(3):1450–1466
Zhu JY, Oh E, Wang T, Wang ZY (2016) TOC1-PIF4 interaction mediates the circadian gating of thermoresponsive growth in Arabidopsis. Nat Commun 7:13692
Author information
Authors and Affiliations
Contributions
Omics ChIP-seq and RNA-seq retrieval and data analysis were done by SK and AvD. Data interpretation and figure preparation were done by US and the main text by SvdK and US.
Corresponding author
Ethics declarations
Consent to Participate
All authors consent to participate in the publication.
Consent for Publication
All authors and WUR consent for the publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
• DNA-ChIP and RNA-seq meta data analysis shows that co-factors that affect transcriptional activity of PIFs, BZR1, and HY5 and MYCs are under (potential) transcriptional feedback control by these same transcription factors, revealing novel and redundant feedback in growth and defense at the level of transcription.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koene, S., Shapulatov, U., van Dijk, A.D.J. et al. Transcriptional Feedback in Plant Growth and Defense by PIFs, BZR1, HY5, and MYC Transcription Factors. Plant Mol Biol Rep 41, 59–80 (2023). https://doi.org/10.1007/s11105-022-01351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-022-01351-9