Abstract
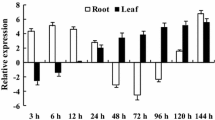
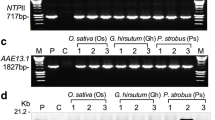
In plants, reactive oxygen species accumulate to a toxic level under various abiotic stresses. Many antioxidant defense systems require NADPH as a principal reducing energy equivalent. However, the source of NADPH and the molecular mechanisms associated with the maintenance of cytoplasmic redox balance are still unknown. The present study describes Vigna NADH kinase (VlNADHK), an enzyme involved in NADPH synthesis and prefers NADH as a diphospho-nicotinamide nucleotide donor. We analyzed the enzymatic activity of a putative cytoplasmic NADH kinase during waterlogging in contrasting mung bean genotypes Vigna luteola (tolerant) and Vigna radiata cv. T44 (susceptible) under pot-culture condition. The tolerant cultivar showed higher enzymatic activity under waterlogging as well as after recovery. Similarly, the transcript level of waterlogging-induced NADHK expression was also studied and found to be upregulated in response to waterlogging in the roots of V. luteola and T44. PCR amplicons of partial and full-length sequences were cloned and sequenced from V. luteola. To the best of our knowledge, this is the first time an ATP-dependent NADH kinase gene has been recognized as a component of waterlogging stress tolerance in legumes. Our study indicated that this cytoplasmic NADH kinase is a primary source of the cytosolic NADPH and might have a role in waterlogging tolerance in legumes.






Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
An Y, Qi L, Wang L (2016) ALA pretreatment improves waterlogging tolerance of fig plants. PLoS One 11:e0147202
Anderson JM, Charbonneau H, Jones HP, McCann RO, Cormier MJ (1980) Characterization of the plant nicotinamide adenine dinucleotide kinase activator protein and its identification as calmodulin. Biochemistry 19:3113–3120
Arnon DI (1949) Copper enzymes in isolated chloroplast: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Berrin JG, Pierrugues O, Brutesco C, Alonso B, Montillet JL, Roby D, Kazmaier M (2005) Stress induces the expression of AtNADK-1, a gene encoding a NAD(H) kinase in Arabidopsis thaliana. Mol Genet Genomics 273:10–19
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: modeling protein tertiary and quaternary structure using evolutionary information. Nucl Acids Res 42(Web Server issue):W252–W258
Chai MF, Chen QJ, An R, Chen YM, Chen J, Wang XC (2005) NADK2, an Arabidopsis chloroplastic NAD kinase, plays a vital role in both chlorophyll synthesis and chloroplast protection. Plant Mol Biol 59:553–564
Chai MF, Wei PC, Chen QJ, An R, Chen J, Yang S, Wang XC (2006) NADK3, a novel cytoplasmic source of NADPH, is required under conditions of oxidative stress and modulates abscisic acid responses in Arabidopsis. Plant J 47(5):665–674
Delumeau O, Paven M-CM-L, Montrichard F, Laval-Martin DL (2000) Effects of short-term NaCl stress on calmodulin transcript levels and calmodulin dependent NAD kinase activity in two species of tomato. Plant Cell Environ 23:329–336
Dordas C (2015) Nitric oxide and plant hemoglobins improve the tolerance of plants to hypoxia. In: Khan M, Mobin M, Mohammad F, Corpas F (eds) Nitric oxide action in abiotic stress responses in plants, Springer, Cham pp 115–128
Du H, Huang M, Zhang Z, Cheng S (2014) Genome-wide analysis of the AP2/ERF gene family in maize waterlogging stress response. Euphytica. 198:115–126
Gallais S, de Crescenzo MA, Laval-Martin DL (2000) Evidence of active NADP+ phosphatase in dormant seeds of Avena sativa L. J Expt Bot 51:1389–1394
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. John Wiley and Sons, New York, p 680
Grose JH, Joss L, Velick SF, Roth JR (2006) Evidence that feedback inhibition of NAD kinase controls responses to oxidative stress. Proc Natl Acad Sci U S A 103:7601–7606
Grzesiak S, Grzesiak MT, Hura T, Marcińska I, Rzepka A (2013) Changes in root system structure, leaf water potential and gas exchange of maize and triticale seedlings affected by soil compaction. Environ Expt Bot 88:2–10
Grzesiak MT, Szczyrek P, Rut G, Ostrowska A, Hura K, Rzepka A, Hura T, Grzesiak S (2015) Interspecific differences in tolerance to soil compaction, drought and waterlogging stresses among maize and triticale genotypes. J Agron Crop Sci 201:330–343
Harding SA, Oh SH, Roberts DM (1997) Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species. EMBO J 16:1137–1144
Hendry GAF, Price AH (1993) Stress indications: chlorophylls and carotenoids. In: Henry GAF, Grime JP (eds) Methods in comparative plant ecology-a laboratory manual. Chapman & Hall, London, pp 148–152
Hiscox JD, Israelstam GF (1979) A method for extraction of chloroplast from leaf tissue without maceration. Can J Bot 57:1332–1334
Ishikawa K, Yoshimura K, Harada K, Fukusaki E, Ogawa T, Tamoi M, Shigeoka S (2010) AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant Physiol 152:2000–2012
Joshi R, Sahoo KK, Tripathi AK, Kumar R, Gupta BK, Pareek A, Singla-Pareek SL (2018) Knockdown of an inflorescence meristem-specific cytokinin oxidase - OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant, Cell & Environment 41(5):936–946
Kawai S, Murata K (2008) Structure and function of NAD kinase and NADP phosphatase: key enzymes that regulate the intracellular balance of NAD(H) and NADP(H). Biosci Biotechnol Biochem 72:919–930
Kawai S, Fukuda C, Mukai T, Murata K (2005) MJ0917 in archaeon Methanococcus jannaschii is a novel NADP phosphatase/NAD kinase. J BiolChem 280:39200–39207
Knox JP, Dodge AD (1985) Singlet oxygen and plants. Phytochemistry 24:889–896
Kokubun M (2013) Genetic and cultural improvement of soybean for waterlogged conditions in Asia. Field Crops Res 152:3–7
Kumar P, Pal M, Joshi R, Sairam RK (2013) Yield, growth and physiological responses of mung bean [Vigna radiata (L.) Wilczek] genotypes to waterlogging at vegetative stage. Physiol Mol Biol Plants 19:209–220
Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant activity in pigeon pea genotypes. Biol Plant 53:75–84
Kushwaha HR, Joshi R, Pareek A, Singla-Pareek SL (2016) MATH-domain family shows response toward abiotic stress in arabidopsis and rice. Front Plant Sci 7:923
Lekshmy S, Jha SK, Sairam RK (2015) Physiological and molecular mechanisms of flooding tolerance in plants. In: Pandey GK (ed) Elucidation of abiotic stress signaling in plants. Vol 2. Springer, New York, pp 227–242
Li WY, Wang X, Li R, Li WQ, Chen KM (2014) Genome-wide analysis of the NADK gene family in plants. PLoS One 9:e101051
Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R (2015) The EMBL-EBI bioinformatics web and programmatic tools framework. Nucl Acids Res 43:W580–W584
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilisation. Nature 479:419–422
Liu M, Jiang Y (2015) Genotypic variation in growth and metabolic responses of perennial ryegrass exposed to short-term waterlogging and submergence stress. Plant Physiol Biochem 95:57–64
Min XJ, Bartholomew DP (2005) Effects of flooding and drought on ehtylene metabolism, titratable acidity and fruiting of pineapple. Acta Hortic 666:135–148
Nutan KK, Kushwaha HR, Singla-Pareek SL, Pareek A (2017) Transcription dynamics of Saltol QTL localized genes encoding transcription factors, reveals their differential regulation in contrasting genotypes of rice. Funct & Integr Genom 17(1):69–83
Outten CE, Culotta VC (2003) A novel NADH kinase is the mitochondrial source of NADPH in Saccharomyces cerevisiae. EMBO J 22:2015–2024
Patil RS, Gaikwad DK, Joshi S (2016) Effect of herbal extracts and plant growth regulators on photosynthetic pigments of soybean under waterlogged condition. Ind J Appl Res 6:32–33
Paul S, Lombroso PJ (2003) Receptor and nonreceptor protein tyrosine phosphatases in the nervous system. Cell Mol Life Sci 60:2465–2482
Puyang X, An M, Han L, Zhang X (2015) Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poapratensis L.) cultivars. Ecotoxicol Environ Saf 117:96–106
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Expt Bot 52:77–84
Ruiz JM, Sanchez E, Garcıa PC, Lopez-Lefebre LR, Rivero RM, Romero L (2002) Proline metabolism and NAD kinase activity in greenbean plants subjected to cold-shock. Phytochemistry 59:473–478
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci 178:171–177
Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC (2008) Physiology and biochemistry of waterlogging tolerance in plants. Biol Plant 52:401–412
Sairam RK, Kumutha D, Chinnusamy V, Meena RC (2009) Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J Plant Physiol 166:602–616
Sairam RK, Dharmar K, Chinnnusamy V, Lekshmy S, Joshi R, Bhattacharya P (2011a) NADPH oxidase as the source of ROS produced under waterlogging in roots of mung bean. Biol Plant 55:741–746
Sairam RK, Dharmar K, Lekshmy S, Chinnusamy V (2011b) Expression of antioxidant defense genes in mung bean (Vigna radiata L.) roots under water-logging is associated with hypoxia tolerance. Acta Physiol Plant 33:735–744
Sairam RK, Chinnusamy V, Arora A, Bhattacharya P, Joshi R, Trivedi S (2012) Non-symbiotic hemoglobin and nitrate reductase constitute an alternative to fermentation in waterlogging tolerance of mung vean (Vigna radiata (L.) Wilczek). Ind J Plant Physiol 17:93–102
Schmittgen TD, Livak KJ, (2008) Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3(6):1101–1108
Shao G, Cheng X, Liu N, Zhang Z (2016) Effect of drought pretreatment before anthesis and post-anthesis waterlogging on water relation, photosynthesis, and growth of tomatoes. Arch Agron Soil Sci 62:935–946
Shi F, Li Y, Li Y, Wang X (2009) Molecular properties, functions, and potential applications of NAD kinases. Acta Biochim Biophys Sin 41:352–361
Singh DP, Singh BB (2011) Breeding for tolerance to abiotic stresses in mung bean. J Food Legumes 24:83–90
Singh R, Mailloux RJ, Puiseux-Dao S, Appanna VD (2007) Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J Bacteriol 189:6665–6675
Sinha BK, Chhabra ML (2015) Effect of phytohormones on biochemical parameters in Indian mustard under abiotic stress conditions. J Hill Agric 6:193–196
Steel RGD, Torri JH, Dickey DA (1980) Principal and procedures of statistics: a biometrical approach. McGraw HillBook Co.Inc, New York, p 672
Takahara K, Kasajima I, Takahashi H, Hashida SN, Itami T, Onodera H, Toki S, Yanagisawa S, Kawai-Yamada M, Uchimiya H (2010) Metabolome and photochemical analysis of rice plants overexpressing Arabidopsis NAD kinase gene. Plant Physiol 152:1863–1873
Takahashi H, Watanabe A, Tanaka A, Hashida SN, Kawai-Yamada M, Sonoike K, Uchimiya H (2006) Chloroplast NAD kinase is essential for energy transduction through the xanthophyll cycle in photosynthesis. Plant Cell Physiol 47:1678–1682
Takahashi H, Takahara K, Hashida SN, Hirabayashi T, Fujimori T, Kawai-Yamada M, Yamaya T, Yanagisawa S, Uchimiya H (2009) Pleiotropic modulation of carbon and nitrogen metabolism in Arabidopsis plants overexpressing the NAD kinase2 gene. Plant Physiol 151:100–113
Tamoi M, Miyazaki T, Fukamizo T, Shigeoka S (2005) The Calvin cycle in cyanobacteria is regulated by CP12 via the NAD (H)/NADP (H) ratio under light/dark conditions. Plant J 42:504–513
Turner WL, Waller JC, Snedden WA (2005) Identification, molecular cloning and functional characterization of a novel NADH kinase from Arabidopsis thaliana (Thale cress). Biochem J 385:217–223
Van Veen H, Vashisht D, Akman M, Girke T, Mustroph A, Reinen E, Hartman S, Kooiker M, van Tienderen P, Schranz ME, Bailey-Serres J (2016) Transcriptomes of eight Arabidopsis thaliana accessions reveal core conserved, genotype-and organ-specific responses to flooding stress. Plant Physiol 172:668–689
Voesenek LA, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73
Voesenek LA, Sasidharan R, Visser EJ, Bailey-Serres J (2016) Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol 209:39–43
Waller JC, Dhanoa PK, Schumann U, Mullen RT, Snedden WA (2010) Subcellular and tissue localization of NAD kinases from Arabidopsis: compartmentalization of de novo NADP biosynthesis. Planta 231:305–317
Wang X, Li WY, Zhang MM, Gao YT, Liu WT, Li WQ, Muhammad I, Chen KM (2016) Identification and functional analysis of the NADK gene family in wheat. Plant Mol Biol Rep 34:118–135
Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 25:1189–1191
Weatherley PE (1950) Studies in water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol 49:81–87
Wu LL, Zhou CY, Gao YS, Cong YX, Chen KM, Guo WL (2011) Cloning and genetic transformation of OsNADK3 gene in rice. J Nucl Agric Sci 25:0863–0870
Yamamoto Y (1966) NAD kinase in higher plants. Plant Physiol 41:523–528
Yuvaniyama J, Denu JM, Dixon JE, Saper MA (1996) Crystal structure of the dual specificity protein phosphatase VHR. Science 272:1328–1331
Zagdanska B (1990) NAD kinase activity in wheat leaves under water deficit. Acta Biochim Pol 37:385–389
Zhang R (2015) MNADK, a long-awaited human mitochondrion-localized NAD kinase. J Cell Physiol 230:1697–1701
Funding
RKS acknowledges the research funds granted by the Department of Science and Technology, Government of India, and institutional project grants of ICAR.
Author information
Authors and Affiliations
Contributions
RJ and PB performed all the experiments and wrote the manuscript. VC and LS made contributions in designing the experiments and in writing the manuscript. RKS conceived and designed the study and finalized the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, R., Bhattacharya, P., Sairam, R.K. et al. Identification and Characterization of NADH Kinase-3 from a Stress-Tolerant Wild Mung Bean Species (Vigna luteola (Jacq.) Benth.) with a Possible Role in Waterlogging Tolerance. Plant Mol Biol Rep 38, 137–150 (2020). https://doi.org/10.1007/s11105-019-01185-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-019-01185-y