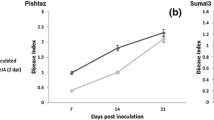
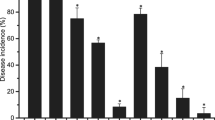
Abstract
The effect of methyl jasmonate (MeJA) treatment on controlling blue mold decay caused by Penicillium expansum in sweet cherry fruit and the possible mechanisms were investigated. The results indicated that fruit treated with MeJA had significantly lower disease incidence and smaller lesion diameter than the control fruit did. The in vitro experiment showed that MeJA transiently inhibited spore germination and germ tube elongation of P. expansum. It is clear that MeJA triggers a priming mechanism in sweet cherry fruit, since only in fruit that had been pretreated with MeJA and then challenged with P. expansum was an enhanced capacity to augment defense responses observed. These augmented responses included enhanced activities of chitinase (CHI) and β-1,3-glucanase (GLU), and increased gene expression levels of catalase (CAT), calmodulin (CaM), GLU, phenylalanine ammonia-lyase (PAL), nonexpressor of pathogenesis-related genes 1 (NPR1-like), and thaumatin-like (THAU). Moreover, MeJA inhibited the increase of activities of polygalacturonase (PG) and pectinmethylesterase (PME). These results suggest that the efficacy of MeJA on controlling blue mold decay in sweet cherry fruit may be related to the transient direct inhibitory effect against the pathogens, suppressed activities of PG and PME, and the priming of defense responses.





Similar content being viewed by others
References
Abeles FB, Bosshart RP, Forrence LE, Habig WH (1971) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol 47:129–134
Ahn IP, Lee SW, Kim MG, Park SR, Hwang DJ, Bae SC (2011) Priming by rhizobacterium protects tomato plants from biotrophic and necrotrophic pathogen infections through multiple defense mechanisms. Mol Cells 32:7–14
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle-dye binding. Anal Biochem 72:248–254
Cantu D, Vicente AR, Greve LC, Dewey FM, Bennett AB, Labavitch JM, Powell ALT (2008) The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc Natl Acad Sci U S A 105:859–864
Cao SF, Zheng YH, Yang ZF, Tang SS, Jin P, Wang KT, Wang XM (2008) Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol Technol 49:301–307
Ceponis MJ, Cappellini RA, Lightner GW (1987) Disorders in sweet cherry and strawberry shipments to the New York market, 1972–1984. Plant Dis 71:472–475
Chang SJ, Puryear J, John C (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19:409–413
Chiasson D, Ekengren SK, Martin GB, Dobney SL, Snedden WA (2005) Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomonas syringae pv. tomato. Plant Mol Biol 58:887–897
Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant–pathogen interactions. Trends Plant Sci 7:210–216
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Droby S, Porat R, Cohen L, Weiss B, Shapio B, Philosoph-Hadas S, Meir S (1999) Suppressing green mold decay in grapefruit with postharvest jasmonate application. J Am Soc Hortic Sci 124:184–188
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
González-Aguilar GA, Tiznado-Hernandez ME, Zavaleta-Gatica R, Martınez-Téllez MA (2004) Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem Biophys Res Commun 313:694–701
Grenier J, Potvin C, Trudel J, Asselin A (1999) Some thaumatin-like proteins hydrolyse polymeric β-1, 3-glucans. Plant J 19:473–480
Guo J, Fang WW, Lu HP, Zhu RY, Lu LF, Zheng XD, Yu T (2014) Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol Technol 88:72–78
Hammerschmidt R (2008) Challenge inoculation reveals the benefits of resistance priming. Physiol Mol Plant Pathol 73:59–60
Harding SA, Oh SH, Roberts DM (1997) Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species. EMBO J 16:1137–1144
Harel MY, Kolton M, Elad Y, Rav-David D, Cytryn E, Ezra D, Graber ER (2011) Induced systemic resistance in strawberry (Fragaria × ananassa) to powdery mildew using various control agents. IOBC/wprs Bull 71:47–51
Janisiewicz WJ, Korsten L (2002) Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441
Jin P, Zheng YH, Tang SS, Rui HJ, Wang CY (2009) Enhancing disease resistance in peach fruit with methyl jasmonate. J Sci Food Agric 89:802–808
Kinkema M, Fan WH, Dong X (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12:2339–2350
Li X, Xu C, Korban SS, Chen K (2010) Regulatory mechanisms of textural changes in ripening fruits. Crit Rev Plant Sci 29:222–243
Lin TP, Liu CC, Chen SW, Wang WY (1989) Purification and characterization of pectinmethylesterase from Ficus awkeotsang Makino achenes. Plant Physiol 91:1445–1453
Roper MC, Greve LC, Warren JG, Labavitch JM, Kirkpatrick BC (2007) Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol Plant Microbe Interact 20:411–419
Sha J (2005) Lipids, lipases and lipid-modifying enzymes in plant disease resistance. Annu Rev Phytopathol 43:229–260
Sun DQ, Lu XH, Hu YL, Li WM, Hong KQ, Mo YW, Cahill DM, Xie JH (2013) Methyl jasmonate induced defense responses increase resistance to Fusarium oxysporum f. sp. cubense race 4 in banana. Sci Hortic 164:484–491
Takabatake R, Karita E, Seo S, Mitsuhara I, Kuchitsu K, Ohashi Y (2007) Pathogen-induced calmodulin isoforms in basal resistance against bacterial and fungal pathogens in tobacco. Plant Cell Physiol 48:414–423
Tian SP, Fan Q, Xu Y, Jiang AL (2002) Effects of calcium on biocontrol activity of yeast antagonists against the postharvest fungal pathogen Rhizopus stolonifer. Plant Pathol 51:352–358
Tonelli ML, Furlan A, Taurian T, Castro S, Fabra A (2011) Peanut priming induced by biocontrol agents. Physiol Mol Plant Pathol 75:100–105
van Hulten M, Pelser M, Van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci U S A 103:5602–5607
Van Loon LC (1985) Pathogenesis-related proteins. Plant Mol Biol 4:111–116
Verhagen BWM, Trotel-Aziz P, Couderchet M, Höfte M, Aziz A (2010) Pseudomonas spp.-induced systemic resistance to Botrytis cinerea is associated with induction and priming of defence responses in grapevine. J Exp Bot 61:249–260
Vilanova L, Wisniewski M, Norelli J, Viñas I, Torres R, Usall J, Phillips J, Droby S, Teixidó (2014) Transcriptomic profiling of apple in response to inoculation with a pathogen (Penicillium expansum) and a non-pathogen (Penicillium digitatum). Plant Mol Biol Rep 32:566–583
Walters D, Walsh D, Newton A, Lyon G (2005) Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95:1368–1373
Wang XL, Xu F, Wang J, Jin P, Zheng YH (2013a) Bacillus cereus AR156 induces resistance against Rhizopus rot through priming of defense responses in peach fruit. Food Chem 136:400–406
Wang XL, Wang J, Jin P, Zheng YH (2013b) Investigating the efficacy of Bacillus subtilis SM21 on controlling Rhizopus rot in peach fruit. Int J Food Microbiol 164:141–147
Wei JM, Ma FW, Shi SG, Qi XD, Zhu XQ, Yuan JW (2010) Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol Technol 56:147–154
Yao HJ, Tian SP (2005) Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol Technol 35:253–262
Yu C, Zeng L, Sheng K, Chen F, Zhou T, Zheng X, Yu T (2014) γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem 159:29–37
Zhou R, Li YF, Yan LP, Xie J (2011) Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) during storage. Food Chem 124:569–575
Zhu Z, Tian SP (2012) Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci Hortic 142:38–43
Zhu X, Caplan J, Mamillapalli P, Czymmek K, Dinesh-Kumar SP (2010) Function of endoplasmic reticulum calcium ATPase in innate immunity-mediated programmed cell death. EMBO J 29:1007–1018
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31172003) and the Jiangsu Provincial Postgraduate Innovation Project of China (CXLX13_266).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Jin, P., Wang, J. et al. Methyl Jasmonate Primed Defense Responses Against Penicillium expansum in Sweet Cherry Fruit. Plant Mol Biol Rep 33, 1464–1471 (2015). https://doi.org/10.1007/s11105-014-0844-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-014-0844-8