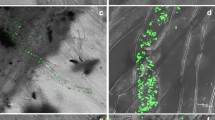
Abstract
A selected strain of rhizobacterium, Pseudomonas putida strain LSW17S (LSW17S), protects tomato plants (Lycopersicon esculentum L. cv. Seokwang) from bacterial speck by biotrophic Pseudomonas syringae pv. tomato strain DC3000 (DC3000) and bacterial wilt by necrotrophic Ralstonia solanacearum KACC 10703 (Rs10703). To investigate defense mechanisms induced by LSW17S in tomato plants, transcription patterns of pathogenesis-related (PR) genes and H2O2 production were analyzed in plants treated with LSW17S and subsequent pathogen inoculation. LSW17S alone did not induce transcriptions of employed PR genes in leaves and roots. DC3000 challenge following LSW17S triggered rapid transcriptions of PR genes and H2O2 production in leaves and roots. Catalase infiltration with DC3000 attenuated defense-related responses and resistance against DC3000 infection. Despite depriving H2O2 production and PR1b transcription by the same treatment, resistance against Rs10703 infection was not deterred significantly. H2O2 is indispensable for defense signaling and/or mechanisms primed by LSW17S and inhibition of bacterial speck, however, it is not involved in resistance against bacterial wilt.
Similar content being viewed by others
References
Ahmad, S., Gordon-Weeks, R., Pickett, J., and Ton, J. (2010). Natural variation in priming of basal resistance: from evolutionary origin to agricultural exploitation. Mol. Plant Pathol. 11, 817–827.
Ahn, I.-P., Lee, S.-W., and Suh, S.-C. (2007). Rhizobacteria-induced priming in Arabidopsis is dependent on ethylene/jasmonic acid and NPR1. Mol. Plant-Microbe Interact. 20, 759–768.
Baker, J.C., O’Neill, N.R., Deahl, K., and Lydon, J. (2002). Continuous production of extracellular antioxidants in suspension cells attenuates the oxidative burst detected in plant microbe interactions. Plant Physiol. Biochem. 40, 641–644.
Barriuso, J., Solano, B.R., and Gutiérrez Mañero, F.J. (2008). Protection against pathogen and salt stress by four plant growthpromoting rhizobacteria isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 98, 666–672.
Benhamou, N., Garand, C., and Goulet, A. (2002). Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ultimum infection in cucumber. Appl. Environ. Microbiol. 68, 4044–4060.
Bittel, P., and Robatzek, S. (2007). Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr. Opin. Plant Biol. 10, 335–341.
Cho, H.J., Hayashi, T., Datta, S.K., Takabayashi, K., Van Uden, J.H., Horner, A., Corr, M., and Raz, E. (2002). IFN-ab promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J. Immunol. 168, 4907–4913.
Choi, G.J., Kim, B.-J., Lim, Y., Choi, K., Yi, Y., Lee, S., Kang, K., Lee, E., Hong, S., Young, J., et al. (2007). Microorganisms against Plasmodiophora brassicae. J. Microbiol. Biotech. 17, 873–877.
Conrath, U., Beckers, G.J.M., Flors, V., García-AgustÍn, P., Jakab, G., Mauch, F., Newman, M.-A., Pieterse, C.M.J., Poinssot, B., Pozo, M.J., et al. (2006). Priming: getting ready for battle. Mol. Plant Microbe Interact. 19, 1062–1071.
Davis, K.R., and Ausubel, F.M. (1989). Characterization of elicitorinduced defense responses in suspension-cultured cells of Arabidopsis. Mol. Plant Microbe Interact. 2, 363–368.
Faize, M., Faize, L., Koike, N., Ishizaka, M., and Ishii, H. (2004). Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology 94, 604–612.
Fidantsef, A.L., Stout, M.J., Thaler, J.S., Duffey, S.S., and Bostock, R.M. (1999). Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesis-related protein P4 in the tomato, Lycopersicon esculentum. Physiol. Mol. Plant Pathol. 54, 97–114.
Houot, V., Etienne, P., Petitot, A.-S., Barbier, S., Blein, J.-P., and Suty, L. (2001). Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 52, 1721–1730.
Hu, X., Bidney, D.L., Yalpani, N., Duvick, J.P., Crasta, O., Folkerts, O., and Lu, G. (2003). Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol. 133, 170–181.
Jetiyanon, K., and Kloepper, J.W. (2002). Mixtures of plant growthpromoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol. Control. 24, 285–291.
Jetiyanon, K., Fowler, W.D., and Kloepper, J.W. (2003). Broadspectrum protection against several pathogens by PGPR mixtures under field conditions in Thailand. Plant Dis. 87, 1390–1394.
Jeun, Y.C., Park, K.S., Kim, C.H., Fowler, W.D., and Kloepper, J.W. (2004). Cytological observations of cucumber plants during induced resistance elicited by rhizobacteria. Biol. Control. 29, 34–42.
Jones, J.D.G., and Dangl, J.L. (2006). The plant immune system. Nature 444, 323–329.
Kandasamy, S., Loganathan, K., Muthuraj, R., Duraisamy, S., Seetharaman, S., Thiruvengadam, R., Ponnusamy, B., and Ramasamy, S. (2009). Understanding the molecular basis of plant growth promotional effect of Pseudomonas fluorescens on rice through protein profiling. Proteome Sci. 7, 47.
Kelman, A. (1954). The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathology 44, 693–695.
Kloepper, J.W., Schroth, M.N., and Miller, T.D. (1980). Effect of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70, 1078–1082.
Kloepper, J.W., Ryu, C.-M., and Zhang, S. (2004). The nature and application of biocontrol microbes: Bacillus spp.-induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94, 1259–1266.
Korolev, N., Rav David, D., and Elad, Y. (2008). The role of phyto hormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana. BioControl. 53, 667–683.
Krause, M.S., De Ceuster, T.J.J., Tiquia, S.M., Michel Jr., F.C., Madden, L.V., and Hoitink, H.A.J. (2003). Isolation and characterization of rhizobacteria from composts that suppress the severity of bacterial leaf spot of radish. Phytopathology 93, 1292–1300.
Lee, S.-W., Ahn, I.-P., Lim, J.-W., and Lee, Y.-H. (2005). Pseudomonas putida strain 17 isolated from replant soil promotes plant growth and inhibits conidial germination of soilborne plant pathogens. Plant Pathol. J. 21, 244–251.
Lin, N.-C., and Martin, G.B. (2005). An avrPto/avrPtoB mutant of Pseudomonas syringae pv. tomato DC3000 does not elicit Ptomediated resistance and is less virulent on tomato. Mol. Plant Microbe Interact. 18, 43–51.
Llorente, F., Muskett, P., Sanchez-Vallet, A., Lopez, G., Ramos, B., Sanchez-Rodriguez, C., Jorda, L., Parker, J., and Molina, A. (2008). Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol. Plant 1, 496–509.
Ludewig, B., Ehl, S., Karrer, U., Odermatt, B., Hengartner, H., and Zinkernagel, R.M. (1998). Dendritic cells efficiently induce protective antiviral immunity. J. Virol. 72, 3812–3818.
Matthes, M., Bruce, T., Ton, J., Verrier, P., Pickett, J., and Napier, J. (2010). The transcriptome of jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta 232, 1163–1180.
Murphy, J.F., Zehnder, G.W., Schuster, D.J., Sikora, E.J., Polston, J.E., and Kloepper, J.W. (2000). Plant growth-promoting rhizobacterial mediated protection in tomato against Tomato mottle virus. Plant Dis. 84, 779–784.
Neuenschwander, U., Vernooij, B., Friedrich, L., Uknes, S., Kessmann, H., and Ryals, J. (1995). Is hydrogen peroxide a second messenger of salicylic acid in systemic acquired resistance? Plant J. 8, 227–233.
Olivain, C., Trouvelot, S., Binet, M.-N., Cordier, C., Pugin, A., and Alabouvette, C. (2003). Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and nonpathogenic strains of Fusarium oxysporum. Appl. Environ. Microbiol. 69, 5453–5462.
Pare, P.W., Farag, M.A., Krishnamachari, V., Zhang, H., Ryu, C.-M., and Kloepper, J.W. (2005). Elicitors and priming agents initiate plant defense responses. Photosynth. Res. 85, 149–159.
Park, D.-S., Landini, S., Graham, M.Y., and Graham, T.L. (2002). Induced distal defence potentiation against Phytophthora sojae in soybean. Physiol. Mol. Plant Pathol. 60, 293–310.
Pozo, M.J., Van Der Ent, S., Van Loon, L.C., and Pieterse, C.M. (2008). Transcription factor MYC2 is involved in priming for enhanced defense during rhizobacteria-induced systemic resistance in Arabidopsis thaliana. New Phytol. 180, 511–523.
Pradhanang, P.M., Ji, P., Momol, M.T., Olson, S.M., Mayfield, J.L., and Jones, J.B. (2005). Application of acibenzolar-S-methyl enhan-ces host resistance in tomato against Ralstonia solanacearum. Plant Dis. 89
Raupach, G.S., and Kloepper, J.W. (1998). Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88, 1158–1164.
Ren, D., Yang, H., and Zhang, S. (2002). Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 277, 559–565.
Ryu, C.-M., Farag, M.A., Hu, C.-H., Reddy, M.S., Kloepper, J.W., and Pare, P.W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134, 1017–1026.
Saile, E., McGarvey, J.A., Schell, M.A., and Denny, T.P. (1997). Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology 87, 1264–1271.
Serbina, N.V., Jia, T., Hohl, T.M., and Pamer, E.G. (2008). Monocytemediated defense against microbial pathogens. Ann. Rev. Immunol. 26, 421–452.
Siegrist, J., Orober, M., and Buchenauer, H. (2000). b-aminobutyric acid-mediated enhancement of resistance in tobacco to Tobacco mosaic virus depends on the accumulation of salicylic acid. Physiol. Mol. Plant Pathol. 56, 95–106.
Takabatake, R., Karita, E., Seo, S., Mitsuhara, I., Kuchitsu, K., and Ohashi, Y. (2007). Pathogen-induced calmodulin isoforms in basal resistance against bacterial and fungal pathogens in tobacco. Plant Cell Physiol. 48, 414–423.
Van der Ent, S., Verhagen, B.W.M., Van Doorn, R., Bakker, D., Verlaan, M.G., Pel, M.J.C., Joosten, R.G., Proveniers, M.C.G., Van Loon, L.C., Ton, J., et al. (2008). MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol. 146, 1293–1304.
Van der Ent, S., Van Hulten, M., Pozo, M.J., Czechowski, T., Udvardi, M.K., Pieterse, C.M., and Ton, J. (2009). Priming of plant innate immunity by rhizobacteria and b-aminobutyric acid: differences and similarities in regulation. New Phytol. 183, 419–431.
van Loon, L.C., Bakker, P.A.H.M., and Pieterse, C.M.J. (1998). Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483.
van Loon, L.C., Bakker, P.A.H.M., van der Heijdt, W.H.W., Wendehenne, D., and Pugin, A. (2008). Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Mol. Plant Microbe Interact. 21, 1609–1621.
Van Wees, S.C.M., Van der Ent, S., and Pieterse, C.M.J. (2008). Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 11, 443–448.
Verhagen, B.W.M., Glazebrook, J., Zhu, T., Chang, H.-S., van Loon, L.C., and Pieterse, C.M.J. (2004). The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol. Plant Microbe Interact. 17, 895–908.
Verhagen, B.W.M., Trotel-Aziz, P., Couderchet, M., Höfte, M., and Aziz, A. (2010). Pseudomonas spp.-induced systemic resistance to Botrytis cinerea is associated with induction and priming of defence responses in grapevine. J. Exp. Bot. 61, 249–260.
Wang, Y., Ohara, Y., Nakayashiki, H., Tosa, Y., and Mayama, S. (2005). Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol. Plant Microbe Interact. 18, 385–396.
Whipps, J.M. (2001). Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 52, 487–511.
Wohlgemuth, H., Mittelstrass, K., Kschieschan, S., Bender, J., Weigel, H.-J., Overmyer, K., Kangasjarvi, J., Sandermann, H., and Langebartels, C. (2002). Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 25, 717–726.
Yang, J.W., Yu, S.H., and Ryu, C.-M. (2009). Priming of defenserelated genes confers root-colonizing Bacilli-elicited induced systemic resistance in pepper. Plant Pathol. J. 25, 389–399.
Yedidia, I., Shoresh, M., Kerem, Z., and Chet, I. (2003). Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperel-lum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 69, 7343–7353.
Zhang, S., Reddy, M.S., and Kloepper, J.W. (2004). Tobacco growth enhancement and blue mold disease protection by rhizobacteria: Relationship between plant growth promotion and systemic disease protection by PGPR strain 90–166. Plant Soil 262, 277–288.
Zhang, H., Xie, X., Kim, M.-S., Kornyeyev, D.A., Holaday, S., and Paré, P.W. (2008). Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 56, 264–273.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ahn, IP., Lee, SW., Kim, M.G. et al. Priming by rhizobacterium protects tomato plants from biotrophic and necrotrophic pathogen infections through multiple defense mechanisms. Mol Cells 32, 7–14 (2011). https://doi.org/10.1007/s10059-011-2209-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-011-2209-6