Abstract
Aims
One of the most important questions of our time is how ecosystems will be transformed by climate change. Here, we used a five-year field experiment to investigate the effects of climate warming on the cover and function of a sub-Arctic alpine ecosystem in the highlands of Iceland dominated by biocrust, mosses and vascular plants.
Methods
We used Open Top Chambers (OTCs) to simulate warming; standard surface and Normalised Difference Vegetation Index (NDVI) analyses to measure plant cover and function; gas analyzers to monitor biocrust respiration; and the Tea Bag Index approach to estimate mass loss, decomposition and soil carbon stabilization rates.
Results
Contrary to our initial hypothesis of warming accelerating an ecological succession of plants growing on biocrust, we observed a warming-induced decreased abundance of vascular plants and mosses —possibly caused by high temperature summer peaks that resemble heat waves— and an increase in the cover of biocrust. The functional responses of biocrust to warming, including increased litter mass loss and respiration rates and a lower soil carbon stabilization rates, may suggest climate-driven depletion of soil nutrients in the future.
Conclusion
It remains to be studied how the effects of warming on biocrusts from high northern regions could interact with other drivers of ecosystem change, such as grazing; and if in the long-term global change could favor the growth of vascular plants on biocrust in the highlands of Iceland and similar ecosystems. For the moment, our experiment points to a warming-induced increase in the cover and activity of biocrust.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological soil crust, often termed biocrust, is a surface system composed of soil particles held together by diverse biological communities, including bryophytes, algae, lichens, fungi and bacteria (Belnap et al. 2016; Bowker et al. 2018; Weber et al. 2022). Despite its small thickness of only a few millimeters to centimeters (Weber et al. 2022), biocrusts are hotspots of microbial abundance (Yoshitake et al. 2018) and diversity (Steven et al. 2013) and play multiple ecosystem functions, including carbon (C) and nitrogen (N) fixation (Elbert et al. 2012), regulation of dust emissions (Rodriguez-Caballero et al. 2022), mediation of vertical and horizontal water flows (Eldridge et al., 2020), and in many cases facilitation of plant establishment and growth (Aradottir and Halldorsson 2018; Zhang et al. 2016; Breen and Levesque 2006).
Biocrust surfaces cover ca. 12% of the terrestrial surface and are projected to decrease under all Representative Concentration Pathways (RCPs) warming scenarios: by ca. -25% in the most optimistic RCP2.6, and by as much as -40% in the severe RCP8.5 (Rodriguez-Caballero et al. 2018). Widespread losses of biocrust cover caused by climate warming would inherently mean widespread losses of its ecological functions.
Much of what is known about the ways biocrusts respond to environmental change comes from long-term field experiments, such as those carried out with open top chambers (OTCs; Hollister et al. 2023). These experiments have shown that OTC-simulated warming, which often causes drying (Salazar et al. 2020), can affect both biocrust cover (Ladrón de Guevara et al. 2018; Lafuente et al. 2018; Maestre et al. 2015) and function (García‐Palacios et al., 2018; Hu et al. 2020; Ladrón de Guevara et al. 2014; Raggio et al. 2023). Most of this research has been concentrated in mid-latitude drylands (e.g., García‐Palacios et al., 2018; Hu et al. 2020; Ladrón de Guevara et al. 2014). Vast areas at high latitudes covered with biocrust (Pushkareva et al. 2016), including polar deserts (Pushkareva et al. 2018; Steven et al. 2013), and wetter environments in alpine and Arctic regions (Salazar et al. 2022), have in comparison been overlooked for various reasons, including logistic challenges. Given the fundamental differences between mid-latitude drylands and high-latitude ecosystems, biocrust from these regions may respond to climate warming in very different ways. To understand how the vast areas of the Arctic and sub-Arctic covered with biocrust could be transformed by climate waring, and the ecological and biogeochemical consequences of such potential transformations, long-term experiments are needed in these regions.
Most in situ warming experiments at high northern latitudes have been conducted in ecosystems predominantly covered by non-crusted surfaces, such as mosses (e.g. Racomitrium lanuginosum; Jónsdóttir et al. 2005) or lichens (e.g. Alectoria ochroleuca; Biasi et al. 2008), or by vascular plants (e.g. Betula nana; Jónsdóttir et al. 2005). Only a handful have been conducted involving ecosystems that are at least partially covered with biocrust (Chapin et al. 1991; Rousk et al. 2018; Sullivan et al. 2008; Wahren et al. 2005). In our study, we investigated the potential effects of warming on biogeochemical and ecological properties of a sub-Arctic alpine biocrust characterized by (but not composed only of) the liverwort Anthelia jurkatzkana (Salazar et al. 2022) —a type of biocrust common in Iceland (Aradottir and Halldorsson 2018; Arnalds 2015; Ortiz-Rivero et al. 2023) and in other high latitude regions (Bakalin et al. 2022; Belland 1983; Smith 1917; Talbot et al. 2018).
Given that in high-latitude ecosystems like our study site many biological processes are limited by low temperatures, we hypothesized that a warming such as that generally created by OTCs (1–2 °C; Hollister et al. 2023) would increase the growth rate of biocrust-forming organisms, but that ultimately it would decrease biocrust cover by accelerating ecological succession i.e. mosses and vascular plants growing on biocrust. Additionally, unless limited by moisture, we expected warming to increase decomposition and biocrust respiration rates. Overall, we can expect that warming within the ranges projected for the next decades (IPCC, 2023) will transform the extent and function of biocrust-dominated subarctic ecosystems. Our five growing seasons study aims at showing the potential path of such transformations.
Methods
Experimental design and abiotic measurements
In June 2018 we set up the Climate Research Unit at Subarctic Temperatures (CRUST) experiment (Fig. S1a; Salazar et al. 2022) in the highlands of Iceland (64°02′11.7"N 19°13′08.2"W; 590 m.a.s.l.). The site is primarily covered with Anthelia jurkatzkana biocrust, mosses, and vascular plants (e.g. Salix herbacea; Fig. S1b), on soils classified as andosol Vitricryands (US Soil Taxonomy) in a cryic soil temperature regime with an abundance of relatively fresh volcanic glass of basaltic and andesitic composition (vitric materials). The area is subjected to periodic volcanic activity and intense (0.1–2 mm yr−1) dust deposition (Arnalds 2015). The soils are coarse grained (sandy loam/loamy sand) with coarse sub-surface tephra layers (volcanic ash) that negatively affect soil water conductivity and storage capacity. Mean annual temperature and precipitation (1971–2000; Icelandic Met Office, 2024) at the site are ca 1.6 °C (Fig. S2) and 1600 mm, respectively. The snow-free season generally starts in late June and ends in October. Flooding is common in June, when the snow melts and the ice under the surface is still frozen. Anthelia jurkatzkana biocrust is often found in this type of late-snow bed ecosystems (Belland 1983; Ottósson et al. 2016; Smáradóttir 2020).
Experimental warming was simulated with OTCs (Hollister et al. 2023) that were built according to the protocols of the International Tundra Experiment (ITEX; Henry and Molau 1997; Henry et al. 2022). We used a randomized block design. First, we visually selected 16 areas of at least 4 × 4 m covered mainly by biocrust and separated by at least 10 m. Then we randomly selected 8 areas (blocks, n = 8). In each of these blocks, we set up two 1.5 m × 1.5 m experimental plots and randomly assigned treatments to them, an OTC and a control (i.e. ambient temperature) plot. The OTCs were left in place all year round.
We measured temperature, light intensity and moisture in OTCs and control plots at different times during five consecutive growing seasons from 2018 to 2022. We measured surface temperature and light intensity every 2 h using HOBO pendant temperature/light data loggers UA-002 64 (Onset Computer, Bourne, Massachusetts, USA). We used one logger per treatment (OTC and control) in 4 of the 8 blocks (n = 4). In the growing seasons of 2018, 2019, 2020 and 2022 we took paired measurements of soil moisture (2–3 cm depth) in all blocks (n = 8) using an ECH2O EC-5 moisture sensor (Decagon Devices, Pullman, WA) attached to a HOBO micro station (Onset Computer, Bourne, Massachusetts, USA).
Aboveground cover and plant community analysis
We estimated percentages of area covered by biocrust, moss and vascular plants in the growing seasons of 2018, 2019, 2021 and 2022 (no measurements in 2020 due to the pandemic). For this, we placed a 50 × 50 cm quadrant, divided in 16 squares of 12.5 × 12.5 cm, in the center of each plot and visually estimated the cover of biocrust, moss and vascular plants (example in Fig. S3). In 2022, we complemented our cover analysis with: 1) Groundbase (SKYE instrument SpectroSense 2 +) measurements of Normalised Difference Vegetation Index (NDVI) as proxy for primary productivity. 2) A detailed plant community analysis using the point intercept method with 25 evenly distributed points within the 50 × 50 cm quadrant. All species intercepted (hits) were recorded for each point along with visual estimates of total vascular plant, moss and litter cover as well as a complete list of vascular plant and moss species providing plot species richness.
Litter decomposition and stabilization factor
To compare the potential fate of organic matter and decomposition rates in the soil, we buried green (Lipton, EAN: 87 22,700 05552 5) and rooibos (Lipton, EAN: 87 22,700 18,843 8) tea bags in OTC and control plots. Tea bags were weighted and buried in the field on July 27th, 2018 and June 25th 2021. To minimize the inherent disturbance of burying and recovering tea bags, we measured mass loss rates only at the beginning and end of the monitoring period. Bags were buried at two depths: at 8 cm, to facilitate comparisons with other studies following the protocol proposed by Keuskamp et al. (2013); under the biocrust (2–3 cm), which is more relevant to our particular study. We tried to minimize disturbance when placing the tea bags. For the 8 cm treatment, we made an incision and placed the tea bags vertically. For the treatment under the biocrust, we placed the tea bags horizontally. We buried 2 subsamples per plot and per depth. The bags were collected on June 5th, 2019 (313 d of incubation) and August 10th, 2022 (411 d incubation), respectively. Notice that the tea bags were incubated for ca. 1 yr (similar to other tundra studies, e.g., Björnsdóttir et al. 2021; von Oppen et al. 2024) and not for 90 days (as in Keuskamp et al. 2013). From the measured mass loss, we calculated litter decomposition rates (k) and stabilization factor (S; an estimate of the fraction of labile compounds that stabilize and become recalcitrant during decomposition) as in Keuskamp et al., (2013). Our decomposition data was submitted to the Tea Bag Index (TBI) network: http://www.teatime4science.org/.
Biocrust respiration
We measured biocrust respiration (RB) in the growing seasons of 2019 (June and July), 2021 (June and July) and 2022 (June and August). RB measurements were not possible in 2018 and 2020 due to logistic reasons and the pandemic, respectively. For that, we installed PVC collars (10 cm depth and diameter) in Anthelia jurkatzkana biocrust patches within the OTCs and control plots, and measured CO2 fluxes periodically using standard procedures with gas analyzers attached to dark soil chambers. All measurements were done approximately in the middle of the day, between 11:00 and 13:00 UTC. Because of instrument availability, we used different gas analyzers in different years. In 2019, we used a Li-6400XT portable system (Li-COR Inc., Lincoln NE, USA); in 2021 a Senseair K-33 ELG sensor (based on a design by Harmon et al. 2015), calibrated with an EGM-4 (PP Systems, Amesbury, Massachusetts, USA); and in 2022 we used an EGM-5 (PP Systems, Amesbury, Massachusetts, USA).
Statistical analysis
We used a combination of statistical approaches: 1) Linear regression (lm function in the R package) to analyze climate trends near our study site (hourly measurements of air temperature at the Vatnsfell station of the Icelandic Meteorological Office, IMO), and the potential relationships between surface light and OTC-warming. 2) Mixed effect models (lmer function), selected based on the Bayesian information criterion (BIC), to analyze differences in litter mass loss, decomposition rates, stabilization factors, and RB. 3) Analysis of variances (aov function) for cover, soil moisture, and NDVI analyses. Surface cover and moisture analyses were further complemented with Tukey Honest Significant Differences (HSD) analyses (TukeyHSD function), to explore potential differences between control and OTC plots in individual years. In the mixed effect models, we analyzed the fixed effects of the OTCs, time and/or tea bag type (depending on the case) and the random effects of the blocks (paired OTC and control plots). To select the mixed effects models with the lowest BICs (i.e. best fit), we used the glmulti package (Calcagno and de Mazancourt 2010). All statistical work was conducted in R, version 4.1.2 (R Core Team 2023).
Results
Simulated warming
Most of the simulated warming happened when sunlight reached the surface (Fig. 1a). In addition to an average warming of 0.83 ± 0.01 °C, OTCs created heat wave-like warming events of up to + 16 °C (Fig. 1b,c). The highest recorded mean (n = 3) surface temperature in our experiment was 50.2 ± 2.0 °C, in the OTC treatment at 15:00 UTC July 19th, 2021 (Fig. 1b). At the same time, surface temperature in control plots was ca. 10 °C lower (40.5 ± 0.4 °C). Maximum surface temperature in control plots was 41.4 ± 0.5 °C (13:00 UTC July 9th, 2021).
Surface light (a), surface temperature (b) and ΔT (c) at the CRUST experiment during five consecutive growing seasons. Data points are average values from functional HOBO pendant temperature/light data loggers. In panel b, the red and black symbols are for OTC and controls, respectively. There were no differences in surface light between OTCs and controls and therefore, for illustration purposes, in panel a we show mean surface light data of control plots only (black symbols). We used red symbols in panel c to illustrate the ΔT between OTC and control plots. At the bottom of panel c, we show the approximate times when cover (C), biocrust respiration (RB), mass loss using the TBI method, and plant community and function (P) analyses took place. The green shading indicates the periods when sunlight reached the surface, based on a visual analysis of the data in panel a
Even though OTC-warming increased with surface light (Fig. S4), which is expected given that OTCs operate like greenhouses, surface temperatures in OTCs remained higher than in control plots even at the beginning of the winter, when surface light went down to zero (likely because of snow cover; Fig. 1). Although OTCs warmed the surface in general, we observed an occasional night-time surface cooling (Figs. 1 and S5) that results from the complex energy balance within the chambers (Hollister et al. 2023; Marion et al. 1997). Finally, as expected, the OTCs had a drying effect. Overall, the soil (2–3 cm depth) in OTCs was 9% drier than in the controls (Fig. S6).
Surface cover
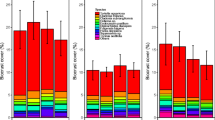
In the summer of 2022, after five growing seasons of OTC-warming, the biocrust, moss, and vascular plant covers were 15% higher, 20% lower and 49% lower in the OTC than in the control plots, respectively (Fig. 2). Vascular plant cover changed substantially between years, both in ambient and OTC-warmed conditions (Table S5-6). Also, in 2022, OTC plots had a higher cover of litter (mostly dead leaves from vascular plants; Fig. S7; Table S7).
Cover of biocrust (black), moss (red) and vascular plants (green) in control (solid lines) and OTC (dashed lines) plots, between 2018 and 2022. Mean values and standard errors. Significance codes: + P < 0.1, * P < 0.05, ** P < 0.01, *** P < 0.001 (anova results in Table S5; Tukey HSD results in Tables S6). n=8
The reduction in moss and vascular plant cover within the OTCs after five consecutive years of simulated warming was not, however, accompanied by a reduction in the number of moss and vascular plant species (Fig. S8) or in their “greenness” (NDVI; Fig. S9 and Table S8). In general, the dominant vascular plant species within OTCs and control plots was Salix herbacea (10% cover), and the dominant moss species was Racomitrium ericoides (21% cover). Other common but low abundant (ca. 1%) vascular plant species were Festuca richardsonii, Bistorta vivipara, and Agrostis stolonifera. We also identified some lichen species, such as Stereocaulon sp. and Cladonia sp., but they were in low abundance and therefore were not included in our surface cover analysis.
Litter mass loss
Overall, mass loss of green and rooibos tea was larger in OTCs than in control plots (Fig. 3 and Table S9). At 2 cm depth, mass loss of green and rooibos tea was 68.2 ± 1.8% and 28.1 ± 0.7% in the control plots, and 71.7 ± 1.4% and 29.6 ± 1.6% in the OTCs, respectively (Fig. 3). Surprisingly, values were similar at 8 cm depth (Fig. 3). Mass loss of green and rooibos tea was greater in 2018–2019 (71.1 ± 1.3% and 30.2 ± 0.8%, respectively) than in 2021–2022 (65 ± 1.7% and 27.1 ± 0.4%, respectively).
Percentage mass loss of litter in green (G) and rooibos (R) tea bags after ca. 1 yr incubation in control (light colors) and OTC (dark colors) plots, on 2018–2019 (left) and 2021–2022 (right), at the CRUST experiment. n = 8 (2018–2019), n = 5 (2021–2022). Significance codes: * P < 0.05, ** P < 0.01, *** P < 0.001 (anova results in Table S9)
We used mass loss data to calculate S and k (Fig. S10) as in Keuskamp et al. (2013). S decreased by 25% with the OTC treatment (0.14 ± 0.03 in comparison to 0.18 ± 0.03 in the control plots) (Fig. S10 and Table S10). In 2018–2019, S was marginally (P < 0.1) higher below the biocrust (0.13 ± 0.02 at 2 cm depth) than in the underlying soil (0.10 ± 0.01 at 8 cm depth; Fig. S10 and Table S11). In 2021–2022, when S was ca. 2 times lower than in 2018–2019, depth did not have a significant effect on S (Table S11). On the other hand, k varied between years and depths, but not with the OTC treatment (Fig. S10 and Table S12). In 2021–2022, k was ca. 10 times lower than in 2018–2019. Also, in 2018–2019 depth had a negative effect on k, meaning that decomposition rates at 8 cm depth were lower than in the topsoil layer (2 cm depth), right below the biocrust (Table S13).
Biocrust respiration (RB)
The best statistical model (-72 BIC) identified with glmulti does not include an effect of the OTC treatment on RB, only differences at the temporal scale (Fig. 4a and Table S14). Since we used different instruments to measure RB, at least part of the observed differences in RB between years could be linked to the performance of the gas analyzers. Because of this, and because of the purpose of our research, we included the OTC treatment in the best statistical model (Table S15) and focus our analysis on the overall differences in paired measurements of RB between control and OTC plots (Fig. 4b). The modified model (Table S15), which had the same performance (-72 BIC) as the original (Table S14), indicated a marginal (P < 0.1) but positive effect of OTCs on RB (Fig. 4b and Table S15).
On the dry day of June 13th, 2019 (Fig. S4), RB was -0.141 ± 0.066 µmol CO2 m−2 s−1 lower in the OTCs than in the control plots. The rest of the measuring times, between 2019 and 2022, RB was larger in the OTCs than in the control plots. The greatest observed difference was on June 30th, 2021, when CO2 emissions from Anthelia jurkatzkana biocrust patches within OTCs were 0.146 ± 0.116 µmol CO2 m−2 s−1 greater than at ambient conditions.
Discussion
As expected, our warming treatment increased average surface temperature by ca. 1 °C, a warming consistent with global trends since 1850 (IPCC, 2023) and local trends near our study site (Fig. S2). Additionally, it created summer, heatwave-like peaks in which surface temperatures inside OTCs were > 10 °C higher than in controls, a common observation in ITEX sites (Bokhorst et al. 2013; Hollister et al. 2023). Our initial hypothesis of a warming-induced acceleration of ecological succession was based on the expected increase in average surface temperatures. However, our findings suggest that ecosystem responses at the CRUST experiment were driven to a large extent by the simulated summer heat pulses.
Heatwaves, which are becoming more frequent in the high north (Dobricic et al. 2020), can be a major source of stress for tundra plants like Salix polaris (Jónsdóttir et al. 2023) and closely related species (Marchand et al. 2005), possibly including S. herbacea, the dominant vascular plant in our study site. Contrary to our hypothesis of vascular plants growing on biocrust, we observed net decreases of vascular plant cover and increases of biocrust cover. It is important to notice that for the period 2018–2021, when we estimated cover using the quadrat method (Fig. S2), an increase of biocrust cover means an increase of the visible biocrust. It is possible that during this period the observed increase of biocrust cover was because of reduction in mosses and vascular plants, thus exposing more of the existing biocrust. In a transplant experiment in the Colorado Plateau (Antoninka et al. 2022), biocrusts from different sources had higher cover and community stability at the highest and coldest elevation. In this sense, the observed increase of biocrust cover in our experiment between 2018 and 2021 could be due to the resistance and/or resilience of Anthelia jurkatzkana biocrust to environmental change. On the other hand, the point intercept that we applied in 2022 accounts for biocrust covered by leaves. Therefore, our observation in 2022 of higher biocrust cover in OTCs (60.4 ± 2.7%) than in control plots (52.6 ± 3.6%) indicates a net increase in biocrust cover. It is not rare for biocrust be more resistant to environmental stress than vascular plants (e.g., Condon et al., 2020). In our case, this observation could be at least partially explained by the capacity of biocrust organisms to rapidly adjust their metabolic rates to sudden changes in the environment (Chongfeng et al. 2017).
Surface cover responses at the CRUST experiment may still be transient. In a similar experiment in northern Sweden, Alatalo et al., (2018) found that the effects of simulated warming on the relative abundance of bryophytes and lichens (two common components of biocrust) varied between years five and seven. In their experiment, warming reduced the relative abundance of bryophytes, with most of the change in the first five years. Warming also decreased the relative abundance of lichens, but in that case most of the reduction happened in the last two years of the experiment. Similarly, in our study the effects of OTC on surface cover differed between years and the most conspicuous differences between control and manipulated plots were at the end of the experiment, particularly for the cover of vascular plants. Future analyses at the CRUST experiment will show whether the effects of OTCs on surface cover will stabilize (as in the case of the bryophytes in Alatalo et al. 2018) or will intensify (as in the case of lichen in Alatalo et al. 2018, and as with the cover of vascular plants in our study). Also, in a laboratory experiment we found that as long as water is not limiting, warming (between 0 and 25 °C) can increase N fixation rates in Anthelia biocrust (Salazar et al. 2022). This suggests that as global temperatures continue rising, biocrusts from the highlands of Iceland and similar cold environments may fix more N2 into terrestrial ecosystems, which could, in the long-term, create favorable nutritional conditions for the establishment of vascular plants.
In addition to simulating warming, one well-known caveat of OTCs is that they unintentionally exclude grazers. In the communal highland grazing areas of Iceland, where our experiment is located, sheep grazing is one of the main drivers of ecosystem degradation (Arnalds and Barkarson 2003; Barrio et al. 2018; Marteinsdóttir et al. 2017). At the CRUST site, we have seen higher abundances of palatable species such as Bistorta vivipara in OTCs than in control plots (Gunnlaugsdóttir 2022), which might be an effect of excluding selective grazing rather than of warming. However, instead of an increase in total vascular plant cover within OTCs, which we initially hypothesized and which could have been explained in part by herbivory exclusion, in the last two years of measurements we observed substantial decreases of vascular plant cover. Because of this, we believe that the differences between OTC and control plots in our experiment were caused, to a larger extent, by the environmental manipulation (both warming and drying) rather than by the unintentional grazing exclusion.
Warming may also have changed the relative abundances of the organisms forming the biocrust. A 13 yrs manipulation experiment at the Colorado Plateau showed that an in situ warming of + 2–4 °C with infrared lamps decreased the cover of mosses by 15% (which is in line with our findings) and differentially affected dark (i.e. late successional) and light (i.e. early successional) biocrusts (Phillips et al. 2022). Overall, in that experiment warming resulted in a stagnation of the system in an early successional state dominated by light-pigmented cyanobacteria such as Microcoleus vaginatus, which can have consequences for the functioning of ecosystems. For example, mature biocrusts store more C and N than less developed biocrusts (Agnelli et al. 2021). We know that on a short-term time scale (hours to days), warming can alter the microbial community and function of the Anthelia jurkatzkana biocrust of our study site (Salazar et al. 2022). Similar analyses but at the multiyear scale remain to be done.
Regarding our analysis of biocrust function, we first need to acknowledge one caveat in our k measurements. For logistic reasons, we incubated the tea bags in the field for ca. 1 yr, instead of 90 days as recommended by Keuskamp et al. (2013). This means that when we collected the tea bags the hydrolysable or labile fraction (i.e. the sum of non-polar extraction, water soluble and acid soluble fractions) could have been exhausted or almost exhausted. Since k is calculated based on a direct relationship with the hydrolysable fraction of the rooibos tea, it is possible that the observed lack of effect of the OTCs on k in our experiment was an artifact caused by an underestimation of k values. Also, k decreases with incubation time. Therefore, the differences in k values between years could reflect differences in incubation periods. In this sense, k values from 2018–2019, calculated based on a shorter incubation period than in 2021–2022, and which suggest an expected negative relationship between depth and k (similar to Miralles et al. 2020), are more reliable. In warm drylands, experimental warming can increase decomposition rates by degrading biocrust and therefore increasing soil-litter mixing (Chuckran et al. 2020). We did not see any evidence of biocrust degradation, which could at least partially explain why warming did not have any effect on k rates in our experiment.
In the case of S rates, which are calculated based on a constant hydrolysable fraction of green tea, we have high confidence in our estimates for both measuring periods. Moreover, all tea bags, in control and OTC plots, were under the same conditions for the same period of time, and therefore the observed differences in S between treatments are comparable. Although biocrust effects are often restricted to the top layer of the soil and decrease with depth (Miralles et al. 2020), in our study warming decreased litter mass loss and S both at 2 and 8 cm depth, suggesting a possible “cascading effect” of the biological activity in the biocrust down to deeper layers of the soil. Overall, our study suggests that warming within limits projected for the high north in the coming decades could increase mass loss in and under the biocrust from our site and similar ecosystems, but reduce the capacity of such biocrust to stabilize labile carbon into recalcitrant forms.
Our findings of higher respiration rates, as well as higher litter cover and mass losses, within the OTCs than in control plots, may indicate a warming-induced increase in turnover rates of carbon. Warming generally decreases biocrust respiration in warm drylands (García-Palacios et al. 2018). This is because of higher mortality of biocrust-forming organisms (e.g., lichens) and/or because of microbial thermal acclimation, as indicated by reductions in the activity of extracellular enzymes such as β‐glucosidase (García-Palacios et al. 2018). However, in wetter conditions, such as wet years in semi-arid grasslands (Escolar et al. 2015) or in biocrust-dominated ecosystems regularly covered with snow (Darrouzet-Nardi et al. 2015; this study), warming can increase biocrust respiration rates and ultimately increase carbon losses. A previous study in our site suggests that surface temperature and respiration are unaffected by OTCs for most part of the snow-free season (Gunnlaugsdóttir 2022). However, our interannual comparison of daytime (ca. 11:00–13:00 UTC) biocrust respiration in the peak of the summer (June–August) suggests a marginal, positive effect of the OTC-warming. Overall, this is consistent with a recent synthesis of 56 OTC in-situ experiments located at Arctic and alpine tundra sites that reports an average 30% increase in ecosystem respiration with warming (Maes et al. 2024).
At least part of our observations, such as the negative ΔRB recorded in the dry June of 2019, could have been caused by drying rather than by warming. Mean annual precipitation is projected to increase in Arctic and sub-Arctic regions in the coming decades (IPCC, 2023). However, biocrust-dominated ecosystems such as the one from our experiment will continue experiencing periods of dryness, especially at sites similar to ours where soils have limited water holding capacity and capillary water transfer from sub-surface horizons during droughts is hindered by near-surface coarse tephra layers (i.e. ash; Arnalds 2015). More data is needed to better understand the ways in which changes in moisture and temperature could affect carbon dynamics in the vast areas of the high north covered with biocrust. Our findings point towards warming increasing carbon emissions from biocrust in the highlands of Iceland, which combined with our observations of higher litter mass loss and lower S values in OTCs than controls, could mean future depletion of soil carbon and possibly other soil nutrients (Ferrenberg et al. 2022).
In conclusion, our findings show potential ways in which global warming can affect high-latitude, biocrust-dominated ecosystems similar to our study site. Contrary to our hypothesis of a relatively mild warming favoring the growth of vascular plants on biocrust, our results suggest that summer heat pulses could decrease the cover of vascular plants and increase biocrust cover and activity. Warming-induced biocrust development has been reported in many locations of the Arctic and sub-Arctic, often on bare ground after a glacier retreat (e.g., Breen and Levesque 2006; Yoshitake et al. 2018). In our study, however, the bare ground cover was negligible. What we observed, was an orchestrated response of the different plant-soil components of an understudied type of biocrust habitat. An observation that fills an empty space in the puzzle that is understanding how tundra ecosystems could be further affected by climate change.
Data availability
Our data has been submitted to the data repository Dryad and is available for review at https://datadryad.org/stash/share/kzARpDSC8MrHjXBspYoqf8pTnmvjbNy2gSul7K4GYNk. (DOI:https://doi.org/10.5061/dryad.tht76hf6r). This is a temporary link to share during the during the peer-review process. If the manuscript is accepted for publication, the dataset will be available to the general public after the publication of the article.
Abbreviations
- Biocrust:
-
Biological soil crust
- CRUST:
-
Climate Research Unit at Subarctic Temperatures
- IMO:
-
Icelandic Meteorological Office.
- ITEX:
-
International Tundra Experiment
- k:
-
Decomposition rates
- NDVI:
-
Normalised Difference Vegetation Index
- OTC:
-
Open Top Chamber
- TBI:
-
Tea Bag Index
- RB :
-
Biocrust respiration
- RCPs:
-
Representative Concentration Pathways
- S:
-
Stabilization factor
References
Agnelli A, Corti G, Massaccesi L, Ventura S, D’Acqui LP (2021) Impact of biological crusts on soil formation in polar ecosystems. Geoderma 401:115340
Alatalo JM, Jägerbrand AK, Molau U (2018) Seven years of experimental warming and nutrient addition causes decline of bryophytes and lichens in alpine meadow and heath communities. PeerJ PrePrints 2:e672v2. https://doi.org/10.7287/peerj.preprints.672v2
Antoninka A, Chuckran PF, Mau RL, Slate ML, Oliver MJ, Stark LR, Bowker MA (2022) Responses of biocrust and associated soil bacteria to novel climates are not tightly coupled. Front Microbiol 13:821860. https://doi.org/10.3389/fmicb.2022.821860
Aradottir AL, Halldorsson G (2018) Colonization of woodland species during restoration: seed or safe site limitation? Restor Ecol 26:S73–S83. https://doi.org/10.1111/rec.12645
Arnalds Ó (2015) The soils of Iceland. Springer Netherlands, Dordts.
Arnalds O, Barkarson BH (2003) Soil erosion and land use policy in Iceland in relation to sheep grazing and government subsidies. Environ Sci Policy 6(1):105–113. https://doi.org/10.1016/S1462-9011(02)00115-6
Bakalin VA, Klimova KG, Karpov EA, Bakalin DA, Choi SS (2022) Liverworts of the South Kamchatka Nature Park: Survival in Active Volcanism Land. Diversity 14(9):722. https://doi.org/10.3390/d14090722
Barrio IC, Hik DS, Thórsson J, Svavarsdóttir K, Marteinsdóttir B, Jónsdóttir IS (2018) The sheep in wolf’s clothing? R ecognizing threats for land degradation in Iceland using state-and-transition models. Land Degrad Dev 29(6):1714–1725. https://doi.org/10.1002/ldr.2978
Belland RJ (1983) A late snow bed bryophyte community in western Newfoundland, Canada. Canad J Bot 61(1):218–223. https://doi.org/10.1139/b83-024
Belnap J, Weber B, Büdel B (2016) Biological soil crusts as an organizing principle in drylands. Springer International Publishing, part of Springer Nature 2016. https://doi.org/10.1007/978-3-319-30214-0
Biasi C, Meyer H, Rusalimova O, Hämmerle R, Kaiser C, Baranyi C, Daims H, Lashchinsky N, Barsukov P, Richter A (2008) Initial effects of experimental warming on carbon exchange rates, plant growth and microbial dynamics of a lichen-rich dwarf shrub tundra in Siberia. Plant Soil 307:191–205. https://doi.org/10.1007/s11104-008-9596-2
Björnsdóttir K, Barrio IC, Jónsdóttir IS (2021) Long-term warming manipulations reveal complex decomposition responses across different tundra vegetation types. Arct Sci 8(3):979–991. https://doi.org/10.1139/as-2020-0046
Bokhorst S, Huiskes AD, Aerts R, Convey P, Cooper EJ, Dalen L, Erschbamer B, Gudmundsson J, Hofgaard A, Hollister RD, Johnstone J, Jónsdóttir IS, Lebouvier M, Vijver BV, Wahren CH, Dorrepaal E (2013) Variable temperature effects of Open Top Chambers at polar and alpine sites explained by irradiance and snow depth. Glob Change Biol 19(1):64–74. https://doi.org/10.1111/gcb.12028
Bowker MA, Reed SC, Maestre FT, Eldridge DJ (2018) Biocrusts: the living skin of the earth. Plant Soil 429:1–7. https://doi.org/10.1007/s11104-018-3735-1
Breen K, Levesque E (2006) Proglacial succession of biological soil crusts and vascular plants: biotic interactions in the High Arctic. Botany 84(11):1714–1731. https://doi.org/10.1139/B06-131
Calcagno V, de Mazancourt C (2010) glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw 34:1–29. https://doi.org/10.18637/jss.v034.i12
Chapin DM, Bliss LC, Bledsoe LJ (1991) Environmental regulation of nitrogen fixation in a high arctic lowland ecosystem. Canad J Bot 69(12):2744–2755. https://doi.org/10.1139/b91-345
Chongfeng B, Chun W, Yongsheng Y, Li Zhang A (2017) Physiological responses of artificial moss biocrusts to dehydration-rehydration process and heat stress on the Loess Plateau, China. J Arid Land 9(3):419–431. https://doi.org/10.1007/s40333-017-0057-8
Chuckran PF, Reibold R, Throop HL, Reed SC (2020) Multiple mechanisms determine the effect of warming on plant litter decomposition in a dryland. Soil Biol Biochem 145:107799. https://doi.org/10.1016/j.soilbio.2020.107799
Condon LA, Bradford JB, Coates PS (2020) Biological soil crusts are more prevalent in warmer and drier environments within the Great Basin ecoregion: implications for managing annual grass invasion. Restor Ecol e14150. https://doi.org/10.1111/rec.14150
Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J (2015) Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 126:363–378. https://doi.org/10.1007/s10533-015-0163-7
Dobricic S, Russo S, Pozzoli L, Wilson J, Vignati E (2020) Increasing occurrence of heat waves in the terrestrial Arctic. Environ Res Lett 15(2):024022. https://doi.org/10.1088/1748-9326/ab6398
Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5(7):459–462. https://doi.org/10.1038/ngeo1486
Eldridge DJ, Reed S, Travers SK, Bowker MA, Maestre FT, Ding J, Havrilla C, Rodriguez-Caballero E, Barger N, Weber B, Antoninka A, Belnap J, Chaudhary B, Faist A, Ferrenberg S, Huber-Sannwald E, Issa OM, Zhao Y, Zhao Y (2020) The pervasive and multifaceted influence of biocrusts on water in the world’s drylands. Glob Change Biol 26(10):6003–6014
Escolar C, Maestre FT, Rey A (2015) Biocrusts modulate warming and rainfall exclusion effects on soil respiration in a semi-arid grassland. Soil Biol Biochem 80:9–17. https://doi.org/10.1016/j.soilbio.2014.09.019
Ferrenberg S, Tucker CL, Reibold R, Howell A, Reed SC (2022) Quantifying the influence of different biocrust community states and their responses to warming temperatures on soil biogeochemistry in field and mesocosm studies. Geoderma 409:115633. https://doi.org/10.1016/j.geoderma.2021.115633
García-Palacios P, Escolar C, Dacal M, Delgado-Baquerizo M, Gozalo B, Ochoa V, Maestre FT (2018) Pathways regulating decreased soil respiration with warming in a biocrust-dominated dryland. Glob Change Biol 24(10):4645–4656. https://doi.org/10.1111/gcb.14399
Gunnlaugsdóttir EG (2022) Experimental warming of subarctic biocrust: temporal responses of soil respiration and plant community. EnCHiL MSc thesis. University of Helsinki and Agricultural University of Iceland
Harmon TC, Dierick D, Trahan N, Allen MF, Rundel PW, Oberbauer SF, Schwendenmann L, Zelikova TJ (2015) Low-cost soil CO2 efflux and point concentration sensing systems for terrestrial ecology applications. MEE 6(11):1358–1362. https://doi.org/10.1111/2041-210X.12426
Henry GH, Hollister RD, Klanderud K, Björk RG, Bjorkman AD, Elphinstone C, Jónsdóttir IS, Molau U, Petraglia A, Oberbauer SF, Rixen C, Wookey PA (2022) The International Tundra Experiment (ITEX): 30 years of research on tundra ecosystems. Arct Sci 8(3):550–571. https://doi.org/10.1139/as-2022-0041
Henry GHR, Molau U (1997) Tundra plants and climate change: the International Tundra Experiment (ITEX). Glob Change Biol 3(S1):1–9. https://doi.org/10.1111/j.1365-2486.1997.gcb132.x
Hollister RD, Elphinstone C, Henry GH, Bjorkman AD, Klanderud K, Björk RG, Björkman MP, Bokhorst S, Carbognani M, Cooper EJ, Dorrepaal E, Elmendorf SC, Fetcher N, Gallois EC, Guðmundsson J, Healey NC, Jónsdóttir IS, Klarenberg IJ, Oberbauer SF, Macek P, May JL, Mereghetti M, Molau U, Petraglia A, Rinnan R, Rixen C, Wookey PA (2023) A review of open top chamber (OTC) performance across the ITEX Network. Arct Sci 9(2):331–344. https://doi.org/10.1139/as-2022-0030
Hu R, Wang XP, Xu JS, Zhang YF, Pan YX, Su X (2020) The mechanism of soil nitrogen transformation under different biocrusts to warming and reduced precipitation: From microbial functional genes to enzyme activity. Sci Total Environ 722:137849. https://doi.org/10.1016/j.scitotenv.2020.137849
IPCC (2023) Sections. In: Core Writing Team, H Lee, J Romero (eds.) Climate change 2023: synthesis report. Contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change . IPCC, Geneva, Switzerland, pp. 35–115. https://doi.org/10.59327/IPCC/AR6-9789291691647
Jónsdóttir IS, Halbritter AH, Christiansen CT, Althuizen IH, Haugum SV, Henn JJ, Björnsdóttir K, Maitner BS, Malhi Y, Michaletz ST, Roos RE, Klanderud K, Lee H, Enquist BJ, Vandvik V (2023) Intraspecific trait variability is a key feature underlying high Arctic plant community resistance to climate warming. Ecol Monogr 93(1):e1555. https://doi.org/10.1002/ecm.1555
Jónsdóttir IS, Magnusson B, Gudmundsson J, Elmarsdottir A, Hjartarson H (2005) Variable sensitivity of plant communities in Iceland to experimental warming. Glob Change Biol 11(4):553–563. https://doi.org/10.1111/j.1365-2486.2005.00928.x
Keuskamp JA, Dingemans BJ, Lehtinen T, Sarneel JM, Hefting MM (2013) Tea Bag Index: a novel approach to collect uniform decomposition data across ecosystems. MEE 4(11):1070–1075. https://doi.org/10.1111/2041-210X.12097
Ladrón de Guevara M, Gozalo B, Raggio J, Lafuente A, Prieto M, Maestre FT (2018) Warming reduces the cover, richness and evenness of lichen-dominated biocrusts but promotes moss growth: insights from an 8 yr experiment. New Phytol 220(3):811–823. https://doi.org/10.1111/nph.15000
Ladrón de Guevara M, Lázaro R, Quero JL, Ochoa V, Gozalo B, Berdugo M, Uclés O, Escolar C, Maestre FT (2014) Simulated climate change reduced the capacity of lichen-dominated biocrusts to act as carbon sinks in two semi-arid Mediterranean ecosystems. Biodivers Conserv 23:1787–1807. https://doi.org/10.1007/s10531-014-0681-y
Lafuente A, Berdugo M, Ladron de Guevara M, Gozalo B, Maestre FT (2018) Simulated climate change affects how biocrusts modulate water gains and desiccation dynamics after rainfall events. Ecohydrology 11(6):e1935. https://doi.org/10.1002/eco.1935
Maes S, Dietrich J, Midolo G, Schwieger S, Kummu M, Vandvik V, Aerts R, Althuizen I, Biasi C, Björk RG, Böhner H, Carbognani M, Chiari G, Christiansen CT, Clemmensen KE, Cooper EJ, Cornelissen H, Elberling B, Faubert P, Fetcher N, Forte T, Gaudard J, Gavazov K, Guan Z, Guðmundsson J, Gya R, Hallin S, Hansen BB, Haugum SV, He J, Hicks Pries C, Hovenden M, Jalava M, Jónsdóttir IS, Juhanson J, Jung JY, Kaarlejärvi E, Kwon M, Lamprecht R, Le Moullec M, Lee H, Marushchak ME, Michelsen A, Munir T, Myrsky E, Nielsen CS, Nyberg M, Olofsson J, Óskarsson H, Parker TC, Pedersen EP, Petit Bon M, Petraglia A, Raundrup K, Ravn NR, Rinnan R, Rodenhizer H, Ryde I, Schmidt NM, Schuur T, Sjogersten S, Stark S, Strack M, Tang J, Tolvanen A, Töpper JP, Väisänen M, van Logtestijn R, Voigt C, Walz J, Weedon J, Yang Y, Ylänne H, Björkman MP, Sarneel J, Dorrepaal E (2024) Environmental drivers of increased ecosystem respiration in a warming tundra. Nature. https://doi.org/10.1038/s41586-024-07274-7
Maestre FT, Escolar C, Bardgett RD, Dungait JA, Gozalo B, Ochoa V (2015) Warming reduces the cover and diversity of biocrust-forming mosses and lichens, and increases the physiological stress of soil microbial communities in a semi-arid Pinus halepensis plantation. Front Microbiol 6:865. https://doi.org/10.3389/fmicb.2015.00865
Marchand FL, Mertens S, Kockelbergh F, Beyens L, Nijs I (2005) Performance of High Arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Glob Change Biol 11(12):2078–2089. https://doi.org/10.1111/j.1365-2486.2005.01046.x
Marion GM, Henry GHR, Freckman DW, Johnstone J, Jones G, Jones MH, Lévesque E, Molau U, Mølgaard P, Parsons AN, Svoboda J, Virginia RA (1997) Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob Change Biol 3(S1):20–32. https://doi.org/10.1111/j.1365-2486.1997.gcb136.x
Marteinsdóttir B, Barrio IC, Jónsdóttir IS (2017) Assessing the ecological impacts of extensive sheep grazing in Iceland. IAS 30:55–72
Miralles I, Soria R, Lucas-Borja ME, Soriano M, Ortega R (2020) Effect of biocrusts on bacterial community composition at different soil depths in Mediterranean semi-arid ecosystems. Sci Total Environ 733:138613. https://doi.org/10.1016/j.scitotenv.2020.138613
von Oppen J, Assmann JJ, Bjorkman AD, Treier UA, Elberling B, Normand S (2024) Microclimate explains little variation in year-round decomposition across an Arctic tundra landscape. Nord J Bot e04062:e04062. https://doi.org/10.1111/njb.04062
Ortiz-Rivero J, Garrido-Benavent I, Heiðmarsson S, de Los Ríos A (2023) Moss and liverwort covers structure soil bacterial and fungal communities differently in the Icelandic highlands. Microb Ecol: 1–16. https://doi.org/10.1007/s00248-023-02194-x
Ottósson JG, Sveinsdóttir A, Harðardóttir M (2016) Vistgerðir á Íslandi. Fjölrit Náttúrufræðistofnunar 54. Garðabær: Náttúrufræðistofnun Íslands (Icelandic Institute of Natural History). Rafræn útgáfa leiðrétt í maí 2018. Icelandic with English summary. https://utgafa.ni.is/fjolrit/Fjolrit_54.pdf
Phillips ML, McNellis BE, Howell A, Lauria CM, Belnap J, Reed SC (2022) Biocrusts mediate a new mechanism for land degradation under a changing climate. Nat Clim Change 12(1):71–76. https://doi.org/10.1038/s41558-021-01249-6
Pushkareva E, Johansen JR, Elster J (2016) A review of the ecology, ecophysiology and biodiversity of microalgae in Arctic soil crusts. Polar Biol 39:2227–2240. https://doi.org/10.1007/s00300-016-1902-5
Pushkareva E, Pessi IS, Namsaraev Z, Mano MJ, Elster J, Wilmotte A (2018) Cyanobacteria inhabiting biological soil crusts of a polar desert: Sør Rondane Mountains, Antarctica. Syst Appl Microbiol 41(4):363–373. https://doi.org/10.1016/j.syapm.2018.01.006
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna (Austria). https://www.R-project.org. Last. Accessed 7 Mar 2024
Raggio J, Pescador DS, Gozalo B, Ochoa V, Valencia E, Sancho LG, Maestre FT (2023) Continuous monitoring of chlorophyll a fluorescence and microclimatic conditions reveals warming-induced physiological damage in biocrust-forming lichens. Plant Soil 482(1–2):261–276. https://doi.org/10.1007/s11104-022-05686-w
Rodriguez-Caballero E, Belnap J, Büdel B, Crutzen PJ, Andreae MO, Pöschl U, Weber B (2018) Dryland photoautotrophic soil surface communities endangered by global change. Nat Geosci 11(3):185–189. https://doi.org/10.1038/s41561-018-0072-1
Rodriguez-Caballero E, Stanelle T, Egerer S, Cheng Y, Su H, Canton Y, Belnap J, Andreae MO, Tegen I, Reick CH, Pöschl U, Weber B (2022) Global cycling and climate effects of aeolian dust controlled by biological soil crusts. Nat Geosci 15(6):458–463. https://doi.org/10.1038/s41561-022-00942-1
Rousk K, Sorensen PL, Michelsen A (2018) What drives biological nitrogen fixation in high arctic tundra: moisture or temperature? Ecosphere 9(2):e02117. https://doi.org/10.1002/ecs2.2117
Salazar A, Rousk K, Jónsdóttir IS, Bellenger JP, Andrésson ÓS (2020) Faster nitrogen cycling and more fungal and root biomass in cold ecosystems under experimental warming: a meta-analysis. Ecology 101(2):e02938. https://doi.org/10.1002/ecy.2938
Salazar A, Warshan D, Vasquez-Mejia C, Andrésson ÓS (2022) Environmental change alters nitrogen fixation rates and microbial parameters in a subarctic biological soil crust. Oikos 2022(11):e09239. https://doi.org/10.1111/oik.09239
Smáradóttir RB (2020) Bacterial community structure and function of Anthelia biological soil crust (MS dissertation). University of Iceland
Smith WG (1917) Anthelia: an Arctic-Alpine Plant Association. In: Transactions of the Botanical Society of Edinburgh, vol. 26, No. 1–4, pp. 36–44. Taylor & Francis Group. https://doi.org/10.1080/03746601709469277
Steven B, Lionard M, Kuske CR, Vincent WF (2013) High bacterial diversity of biological soil crusts in water tracks over permafrost in the high arctic polar desert. PLoS ONE 8(8):e71489. https://doi.org/10.1371/journal.pone.0071489
Sullivan PF, Welker JM, Steltzer H, Sletten RS, Hagedorn B, Arens SJ, Horwath JL (2008) Energy and water additions give rise to simple responses in plant canopy and soil microclimates of a high arctic ecosystem. J Geophys Res Biogeosci 113(G3). https://doi.org/10.1029/2007JG000477
Talbot SS, Schofield WB, Váňa J, Talbot SL (2018) Liverworts from Attu Island, Near Islands, Aleutian Islands, Alaska (USA) with comparison to the Commander Islands (Russia). BP 7(2):127–141. https://doi.org/10.17581/bp.2018.07203
Wahren CH, Walker MD, Bret-Harte MS (2005) Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob Change Biol 11(4):537–552. https://doi.org/10.1111/j.1365-2486.2005.00927.x
Weber B, Belnap J, Büdel B, Antoninka AJ, Barger NN, Chaudhary VB, ... Bowker MA (2022) What is a biocrust? A refined, contemporary definition for a broadening research community. Biol Rev 97(5):1768–1785. https://doi.org/10.1111/brv.12862
Yoshitake S, Uchida M, Iimura Y, Ohtsuka T, Nakatsubo T (2018) Soil microbial succession along a chronosequence on a High Arctic glacier foreland, Ny-Ålesund, Svalbard: 10 years’ change. Polar Sci 16:59–67. https://doi.org/10.1016/j.polar.2018.03.003
Zhang Y, Aradottir AL, Serpe M, Boeken B (2016) Interactions of biological soil crusts with vascular plants. In: Weber B (ed) Biological soil crusts: an organizing principle in drylands, 2nd edn. Springer Link, pp 385–406
Acknowledgements
We thank the Icelandic Research Fund for partially funding this work with the grant no. 163336. We thank the staff of the Nordic Master Program in Environmental Changes at Higher Latitudes (EnCHiL), for supporting Eyrún Gyða in her research, including measurements at the CRUST experiment that are included in this article. We thank the Icelandic Meteorological Office and Emmanuel P. Pagneux for their help with meteorological data. Finally, we thank Gideon Asamoah, a fellow of the 2022 cohort of the GRÓ-Land Restoration Training program, for his help with the collection of tea bags in the summer of 2022.
Funding
Much of this work was financed with the 2016 Icelandic Research Fund grant no. 163336.
Author information
Authors and Affiliations
Contributions
Alejandro Salazar, Ingibjörg S. Jónsdóttir and Ólafur Andrésson contributed to the study conception and design. All the authors contributed to material preparation, data collection and/or analysis. The first draft of the manuscript was written by Alejandro Salazar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Stefano Cesco.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salazar, A., Gunnlaugsdóttir, E.G., Jónsdóttir, I.S. et al. Increased biocrust cover and activity in the highlands of Iceland after five growing seasons of experimental warming. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06900-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06900-7