Abstract
Aims
Motivated by recent studies highlighting the active role of plants in influencing the hydraulic properties of the rhizosphere through mucilage exudation, this study explored the effect of plant rhizosphere regulation on the emergent hydraulic properties of the rhizosphere under varying soil texture and moisture levels.
Methods
Maize (Zea mays) plants were cultivated in sand and loam soils exposed to varying moisture levels. Neutron radiography was employed to quantify the profile of water content (\({\theta }_{r})\) around lateral and crown roots. The \({\theta }_{r}\) were used to derive rhizosphere hydraulic indices such as rhizosphere extension (\({d}_{rh}\)), water content at the root-soil interface (\({\theta }_{rs}\)), and water content in the rhizosphere (\({\theta }_{rh}\)). The latter two attributes were normalized by the respective bulk soil water contents (\({\theta }_{rs}^{*}\), \({\theta }_{rh}^{*}\)).
Results
Results showed a higher \({\theta }_{rs}^{*}\), \({\theta }_{rh}^{*}\) and \({d}_{rh}\) in coarse-textured soil versus fine-textured soil. This indicates a more pronounced rhizosphere development in sand compared to loam, possibly via mucilage exudation to maintain better root-soil contact. In contrast, soil water content did not impact the rhizosphere properties of crown roots but impacted the rhizosphere properties of lateral roots. The derived rhizosphere water retention curves revealed a higher water-holding capacity of the rhizosphere in both soils compared to bulk soil.
Conclusions
Our study underscores that soil-grown maize plants dynamically adjust the hydraulic properties of their rhizosphere in response to external factors, primarily aiming to optimize root-soil contact. That leads to a more pronounced rhizosphere in coarser textures, interacting with the soil moisture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil drying plays a crucial role in governing the growth of plants, acting as a major constraint on their access to water and nutrient resources. The extent of this constraint is linked to soil characteristics, including texture, structure, mineralogy, soil organic matter, and pH. The root-soil interface is a critical zone where the contact between the root surface and the liquid phase undergoes a significant reduction as the soil dries, limiting water and nutrient flux from the soil to the roots (Gardner 1991; Carminati and Javaux 2020).
In recent years, attention has been increasingly directed towards root and rhizosphere traits, recognizing their potential to enhance plant access to water and nutrients in limited conditions. Certain traits have received exclusive consideration due to their impact on extending the root surface area in contact with the soil liquid phase. These include root architecture, root branching patterns, root diameter, mycorrhiza symbiosis, root hairs, root exudation, and root mucilage (Comas et al. 2013; Lynch 2013; Zhan et al. 2015).
Mucilage is a rhizosphere trait that has recently gained attention for its potential to improve soil hydraulic properties, particularly the hydraulic contact with the liquid phase in drying soils (Ahmed et al. 2014; Benard et al. 2019; Zarebanadkouki et al. 2019). Mucilage, a polymeric gel primarily secreted by the root tips of many crops, has been associated with various functions, such as influencing the dynamics of nutrients in the rhizosphere (Walker et al. 2003; Hinsinger et al. 2005), maintaining the soil surrounding the root tip wet and hydrated (McCully and Boyer 1997; Ahmed et al. 2014), and acting as a lubricant at the root surface, improving root penetration in soil (Iijima et al. 2004). One additional putative function attributed to mucilage is the facilitation of root water and nutrient uptake (Read et al. 2003; Liebersbach et al. 2004; Carminati et al. 2016; Zarebanadkouki et al. 2019). The beneficial function of mucilage in enhancing root water and nutrient uptake is closely tied to its ability to increase the water-holding capacity of soils, maintaining better liquid phase connectivity within soil pores and improving the hydraulic contact between roots and the surrounding liquid phase (McCully and Boyer 1997; Zarebanadkouki et al. 2019; Benard et al. 2019; 2021). Carminati et al. (2017) explained how the combination of increased viscosity and decreased surface tension of the liquid phase could alter the spatial configuration of the liquid phase within the soil pores, enhancing the connectivity of the liquid phase during soil drying.
Assuming a potential advantageous role of mucilage under soil drying conditions, one would expect adaptive adjustments in the quantity and quality of mucilage exudation by plants. However, it has proven challenging to predict these adaptations from the plant’s perspective. Under water deficit conditions, it is frequently observed that plants reduce their leaf-to-root ratio to prioritize water and nutrient resources over carbon sink activities, leading to an overall reduction in the growth of the root system (Palta and Gregory 1997). This prompts questions about carbon and mucilage exudation to the soil under these conditions. While the C input to the soil via exudation might be reduced due to carbon limitation, the effective concentration per unit of root length (tip) may increase (Holz et al. 2018). This increase can occur either actively, such as strategic adjustments by the plant, or passively, as a result of restricted mobility and the degradation of exudates within soils during drying conditions. Furthermore, plants may strategically fine-tune the physical and chemical composition of their root exudates, such as augmenting mucilage viscosity to reduce its mobility within the soil. This adjustment favors a higher mucilage content at the root-soil interface, achieved through a lower expected diffusion/convection in drying soils (Holz et al. 2018). The localized increase in mucilage concentration at a shorter distance from the root surface enhances the expected contribution of the rhizosphere to water and nutrient uptake, making it more effective. For instance, coarse-textured soils exhibit greater drought stress vulnerability than their fine-textured counterparts due to limited mobility of water, imposed by a lower unsaturated hydraulic conductivity at low potentials (Draye et al. 2010). This soil-centric constraint establishes a multifaceted impact, influencing the availability, mobility, and transport of water and nutrients within soils. However, an unresolved appealing question persists concerning whether plants regulate the hydraulic properties of their rhizosphere by adjusting the quality or quantity of mucilage exudation in response to environmental conditions, such as varying soil textures and moistures. The current state of knowledge faces constraints due to a lack of methodologies capable of directly probing mucilage exudation from soil-grown plant roots (Oburger and Jones 2018). Furthermore, the millimeter-scale nature of the rhizosphere presents technical challenges in sampling and studying its hydraulic properties.
Recently, Nazari et al. (2023) quantified mucilage exudation from aerial nodal roots of maize varieties from different climatic regimes growing in varying soil textures and climates. They demonstrated that the amount of exuded mucilage was significantly impacted by the soil textures experienced by the rest of the root system and the climate. Similarly, Kroener et al. (2018) explored the effects of mucilage extracted from chia seeds on water retention and flow within the soil matrix, revealing a dependency on soil texture and mucilage content. They argued that a higher mucilage content is necessary for effective alterations in water retention and flow in coarse-textured soils, emphasizing the need to establish extensive mucilage networks capable of bridging larger pores inherent in these soils. They suggested that this network formation requires either increased mucilage viscosity from altered physical and chemical properties or an increase of the mucilage content in the liquid phase.
This study aims to investigate how strong plants regulate the emergent hydraulic properties of the rhizosphere under varying soil conditions, specifically soil texture and moisture levels, to optimize the hydraulic contact with the soil. To achieve this objective, we quantified the in-situ profile of soil water content in the rhizosphere of newly emerged root tips. This serves as an emergent proxy for the rhizosphere soil hydraulic properties. Maize (Zea mays) plants were cultivated in soils with two contrasting textures (sand and loam) and exposed to differing soil moistures representing both wet and dry conditions. When plants were 6 weeks old, neutron radiography was employed as a technique to quantitatively visualize water content (\(\theta\)) around the roots. We assume that any modulation in the quantity and quality of mucilage exuded by plant roots would be evident as wetter and more extended rhizosphere around the roots. Plants may aim to enhance their contact with the liquid phase and, consequently, improve access to water and nutrient resources under water-constrained conditions. Subsequently, we hypothesize to observe a more pronounced rhizosphere extension (\({d}_{rh}\)) in water-limited conditions.
Material and methods
Soil and plant preparations
The experiment was conducted in two contrasting soil textures: a loamy soil characterized by a grain size distribution of 33.2% sand, 47.7% silt and 19.1% clay, and a sandy soil derived from dilution of the loamy soil with quartz sand (83.3% sand, 16.7% loam), resulting in a grain size distribution of 88.6%, 8.1%, 3.3% (Vetterlein et al. 2021). Before filling the containers, the soil was air-dried at 60 °C and a nutrient solution was applied to the respective soils as described in Vetterlein et al. (2021). Aluminum containers of the size 30 cm in height by 25 cm in width by 1 cm in thickness were positioned horizontally and uniformly filled with soil from the open side by continuously pouring soil through a sieve (mesh size of 2 mm) to prevent soil layering. The filling procedure resulted in a bulk density of 1.4 g/cm3 in loam and 1.6 g/cm3 in sand. After filling the containers, the soil volumetric water content was adjusted to 10 ± 2% [vol.] and 20 ± 2% [vol.], hereafter called ‘dry’ and ‘wet’ treatments, and was kept constant throughout the growth period. Four replicates were prepared for each treatment.
Pre-germinated maize (Zea mays) seeds of the wild-type genotype B73 were planted in each container at a depth of 1 cm. The seeds were pre-germinated, as follows: the seeds were sterilized in 10% H2O2 (v/v) for 10 min and then placed on a petri dish moist with 0.015 M CaSO4 solution and kept in the dark for 2 days. One seedling per container was cultivated and let grow under controlled conditions at temperatures of 19–24 °C at night/day, relative humidity of 55–65%, and a light intensity of 1100 µmol m-2 s-1 set to a photoperiod of 12 h. During growth conditions, the volumetric water content was regularly adjusted to respective ranges among each treatment by adding water from the top. When plants were 1 week old, the soil surface was covered by a 1 cm thick layer of coarse sand to exclude soil evaporation. Neutron radiography technique was used to monitor the soil water content distribution around the roots of 6-week-old maize plants. At this stage, plants grown in sandy soil had an average transpiration rate of 60 ± 4 g/d and 37 ± 4 g/d when grown under wet and dry conditions, respectively. The average transpiration for plants grown in loamy soil was 20 ± 8 g/d and 14 ± 5 g/d when grown under wet and dry conditions, respectively.
Neutron radiography
Neutron radiography was conducted in the NEUTRA beamline of the Paul-Scherrer Institute in Switzerland. The images were taken with an Andor iKon L camera, with a pixel size of 63 µm/pixel and a spatial resolution of 90 µm. The images had 2047 × 2047 pixels, resulting in a field of view of ca. 13 × 13 cm. Our samples were exposed to the neutron beam 20 s before an image was taken. Considering our field of view, a total of nine overlapping radiographs were needed to image the entire soil domain. Prior to the neutron radiography experiments, plants were kept in the dark for three hours to let water potential across the soil-root system equilibrate. The samples were scanned for three consecutive days to monitor the soil water content distributions around the roots. The soil water content was readjusted to the respective moisture during night-time after scanning the samples. To do so, the containers were weighed, and the missing volume of water was added to several soil regions via a syringe, ensuring a rather constant soil water content over time within the samples.
Image processing
The transmission of the neutron beam through a sample is described by the Beer-Lambert law:
where x and y are the coordinates of the field of view, \(I(x,y)\) is the intensity of the attenuated neutron beam [number of neutrons m−2 s−1], \({I}_{0}(x,y)\) is the intensity of the incident neutron, \({I}_{DC}(x,y)\) is the dark current image (an image recorded by the camera when the beam was off), \({d}_{i}(x,y)\) is the thickness of the ith-material composing the sample [cm], and μi is the neutron attenuation coefficient describing the probability of neutron interactions with the ith-material per unit of thickness [m−1].
For our experimental setup (aluminum containers filled with soil and water), we want to know the water content for each pixel. We are thus not interested in the attenuation contributed by container and soil. This is achieved by replacing \({I}_{0}\) in Eq. (1) by \({I}_{dry}\) which represents an image of the dry sample, i.e., the transmitted beam intensity through a rhizobox filled with dry soil. The Eq. (1) can be rewritten as
where dH2O is the thickness of water and μH2O is the attenuation coefficient of water (μH2O = 2.33 cm−1), which was measured using control samples with a known thickness of H2O. The average soil water content \(\theta (x,y)\) [cm cm−1] was calculated using pixels without roots as
where dtot = 1 cm is the thickness of the inner space of the rhizobox in the beam direction.
The high contrast observed in the radiographs between the roots and the surrounding soil enabled us to segment the roots from their surrounding soil effectively. To overcome the challenges associated with root segmentation, we specifically chose to analyze the growing segments of lateral and crown roots within three days, ensuring that there was no overlap between the selected segments and other root segments. The segmentation was done in ImageJ, using the threshold function to mask the growing root tips. In addition, for sandy soil, we selected roots located at similar depths with comparable water content in their surrounding soil. This selection was crucial because the gradient in water potential due to gravity across soil depth in sandy soil leads to a corresponding gradient in water content over depth.
From the radiograph computed by Eq. 3, we calculated the profile of water content as a function of distance from the root surface \(\theta \left(x\right)\) by applying the Euclidean distance map to the segmented root and averaging \(\theta (x,y)\) in the pixels with a respective distance of \(x\) from the root surface. The calculated profiles were noisy due to interference of neighboring roots, particularly in the case of plants growing in loamy soil due to the heterogeneous nature of this soil. To achieve a smoother soil water content profile, we first calculated the change in soil water content using the images of three successive days as following
where \(\Delta \theta\) is the change in soil water content between 3 successive days, \(I(x,y,t1)\) and \(I(x,y,t3)\) refer to neutron radiographs on day 1 and day 3 of the neutron radiography experiment, respectively. To exclude the change in soil water content in the bulk soil during these three successive days, the change in bulk soil water content was subtracted from these profiles by
where \({\Delta \theta }_{bu}\) is the change in water content in the bulk soil calculated as the average change in soil water content at a distance of 0.4–1 cm from the root surface. Finally, the profile of soil water content as a function of distance from the root surface was calculated as
where \(\theta (x,t1)\) refers to the profile of water content on the first day calculated using Eq. 3. Note that \(\theta (x,t1)\) is expected to be smoother since roots are not yet present in this selected soil region.
As the radiographs obtained are two-dimensional representations resulting from neutron transmission across the sample thickness, the pixels adjacent to the root in the radiographs provide an average water content estimation for the rhizosphere and the soil regions located in front of and behind the rhizosphere. In order to determine the actual water content within the rhizosphere, we made the assumption of radial symmetry in the distribution of water around the roots. Under this assumption, the relationship between the actual water content as a function of radial distance from the root surface \(\theta \left(r\right)\), and the average water content as a function of distance from the root surface \(\overline{\theta }(x)\), was calculated by (more details given by Zarebanadkouki et al. 2016):
where dtot is the inner thickness of the sample, r is the radial distance from the root axis, and x is the apparent distance from the root axis in the radiograph. Equation 7 is derived by changing the Cartesian coordinates into radial coordinates.
Evaluation of water contents in the rhizosphere and bulk soil
To quantify water content in the rhizosphere region and compare it with bulk soil, each calculated soil content profile (Eq. 7) was first fitted with an exponential decay function. From this fitted profile, the rhizosphere characteristics such as the normalized water content in the root-soil interface (\({\theta }_{rs}^{*}\)), the normalized water content in the rhizosphere (\({\theta }_{rh}^{*}\)), and the rhizosphere extension (\({d}_{rh}\)), were obtained. The procedure of calculation of these indices is conceptualized in Fig. 1. For each profile, the average water contents in a region with a distance of 0.4–0.5 cm from the root surface was taken as the average water content in the bulk soil (\({\theta }_{bulk}\), Fig. 1, hatched box (1)). Then, the respective measured water content profile was normalized by its bulk soil water content. The rhizosphere extension \({d}_{rh}\) was calculated as the maximum distance from the root surface, at which the water content was 5% higher than \({\theta }_{bulk}\) (Fig. 1, blue vertical line (2)). Using \({d}_{rh}\), the average water content in the rhizosphere (\({\theta }_{rh}\)) was calculated from the water content profiles, as well as the normalized water content profiles (\({\theta }_{rh}^{*}\), Fig. 1, blue hatched box (3)). Similarly, the average water content in the area from the root surface to the fifth pixel (0.315 mm) was taken as the average water content in the root-soil interface (\({\theta }_{rs}\), Fig. 1, (4)).
Estimation of rhizosphere water retention curve
Measurement and evaluation of the water retention curve of rhizospheric soil are challenging due to the technical difficulties of in-situ soil sampling from such a tiny region in an amount needed for conventional methods of quantifying soil retention curves. We aimed to estimate the rhizosphere water retention curve based on the measured water contents in the bulk soil and the rhizosphere and the available retention curve of the bulk soil. The retention curves for bulk soils were measured using an evaporative method implemented in Hyprop, and their parameters were reported by Vetterlein et al. (2021). We assumed a spatially uniform soil matric potential around the roots since neutron radiography experiments were performed during nighttime when plants were not transpiring. Based on this assumption, soil matric potential was derived from the measured water content in the bulk soil and the available bulk soil retention curve. Then, the rhizosphere water retention curves were derived by plotting the respective \({\theta }_{rh}\) over the matric potentials of the bulk soil. Subsequently, the van Genuchten–Mualem model was fitted to the resulting data pairs to obtain the model parameters of the rhizosphere retention curve.
Statistical analysis
To determine the statistical significance of the treatments (texture, moisture) on the normalized water content in the root-soil interface (\({\theta }_{rs}^{*}\)), the normalized water content in the rhizosphere (\({\theta }_{rh}^{*}\)), and the rhizosphere extension (\({d}_{rh}\)) a two-way ANOVA for each dependent variable was performed using the statsmodels package in Python (Perktold et al. 2024). Data of \({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\) were transformed by taking the exponential data of \({d}_{rh}\) was log-transformed to accomplish a normal distribution of residuals. The two-way ANOVA was followed by a post-hoc Tuckey test. The level of significance was set to α = 0.05.
Results
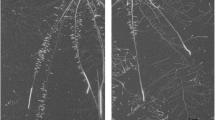
Figure 2a shows the neutron radiography of a 6-week-old maize plant grown in sandy soil subjected to 20% volumetric soil water content on the first day of the neutron radiography experiment. This figure shows water content and root distribution within the soil (i.e., the darker the color map, the wetter the soil). Figure 2b shows growing root tips from day 1 to day 3 obtained from the difference between captured neutron radiographs from day 1 to day 3. In this radiograph, new emerging root tips are distinctly visible as darker regions than the rest (Fig. 2b). For the sake of illustration, we selected and segmented six root tip examples, as shown in Fig. 2c, and presented details of their quantification in Fig. 3.
(a) Neutron radiography image demonstrating the distribution of roots and volumetric soil water content in a 6-week-old maize plant grown in a 30 × 25 × 1 cm aluminum container filled with sandy soil with approximately 20% volumetric soil water content. The image is obtained by stitching nine overlapping radiographs together. Colormap indicates soil water content, with black representing wetter regions. (b) The difference between images captured on day 3 and day 1 highlights the change in water content (i.e., the darker the region, the higher the increase in water content). In this image, pixels containing new emerging roots and their rhizosphere appeared dark due to their higher water content than soil. (c) Six segmented growing root tips (colored in red) from this plant were used as an example to demonstrate the details of our quantification procedure
Figure 3a shows calculated profiles of soil water content as a function of the distance from the root surface for the roots presented in Fig. 2c. \({\theta }_{rh}\) and \({\theta }_{rs}\) were significantly higher than \({\theta }_{bulk}\) of the respective roots (p < 0.05, Fig. 3a). By normalizing the rhizosphere water content profiles by \({\theta }_{bulk}\), the profiles shift together, allowing a comparison of the profiles of different roots (Fig. 3b). On average \({\theta }_{rh}\) of crown roots was 31% and of lateral roots was 26% wetter than the bulk soil.
The \({\theta }_{rs}^{*}\) around newly emerged roots were significantly lower in the loam compared to the sand for both root types (Fig. 4a, b). The moisture treatments had no effect on the \({\theta }_{rs}^{*}\) of newly emerged crown roots, neither in loam nor in sand (Fig. 4a). For the lateral roots, both the soil texture and the soil moisture treatments had a significant effect in loam, where the \({\theta }_{rs}^{*}\) was 1.1 ± 0.03 in the wet treatment compared to 1.24 ± 0.12 in the dry treatment (Fig. 4b). In sand, the pattern was the opposite, with a significantly higher \({\theta }_{rs}^{*}\) in the wet treatment (1.69 ± 0.18), compared to the dry treatment (1.27 ± 0.18), also projected by the significant interaction of texture and moisture. The development of the water content in the whole rhizosphere (\({\theta }_{rh}^{*}\)) showed the same pattern as \({\theta }_{rs}^{*}\) (Fig. 4 c, d). Since it averages over a larger soil volume, the values are lower in the rhizosphere compared to the root-soil interface.
(a) Impact of soil texture and soil water content on \({\theta }_{rs}^{*}\) for all crown roots and in (b) for lateral roots. Plots in (c) and (d) depict the impact of the treatments on \({\theta }_{rh}^{*}\) of crown roots and lateral roots, respectively. Effect of the treatment was tested with a two-factorial ANOVA followed by comparison of means, tested by Tukey-test, significant differences (p < 0.05) are denoted by different letters. The boxplots indicate the median represented by the black line. The boxes extend from the first quartile (25th percentile) to the third quartile (75th percentile) and the whiskers range from the boxes to the widest data point, which lies within 1.5 times the interquartile range. Fliers are data points that fall outside the range delineated by the whiskers
On average, \({d}_{rh}\) of newly developed rhizosphere is estimated to be 0.35 ± 0.19 mm, which corresponds to 0.65-fold the root diameters. The results were determined by soil texture, but the soil moisture had no effect on \({d}_{rh}\) for both root types (Fig. 5). The rhizosphere extended further in the sand compared to the loam, but the interaction of texture and moisture showed no significant effect for crown roots (Fig. 5a). For crown roots, values of \({d}_{rh}\) were 0.33 ± 0.14 mm in loam and 0.42 ± 0.11 mm in sand. The same response of the developing \({d}_{rh}\) to soil texture was observed around lateral roots (Fig. 5b). More specifically, the rhizosphere around lateral roots developed significantly greater in loam in the dry treatment (0.43 ± 0.23 mm), compared to the wet treatment (0.17 ± 0.07 mm, see Fig. 5b). This pattern was the other way around for lateral roots, growing in sand, where \({d}_{rh}\) was significantly higher in the wet (0.54 ± 0.2 mm) compared to the dry treatment (0.28 ± 0.13 mm, Fig. 5b), also reflected by the significant interaction of texture and moisture. Neither crown roots nor lateral roots showed any response to the moisture treatments (Fig. 5a, b).
In Fig. 6, we combined data from both lateral roots and crown roots since we did not observe a significant difference in rhizosphere water content linked to the root type. The results showed that in both soil textures at any soil matric potential, the rhizosphere soils held more water than the bulk soil. The fitted parameters for the bimodal Mualem-van Genuchten model for the bulk soil and the rhizosphere of maize plants are reported in Table 1. The moisture treatments were 10% and 20% volumetric soil water content. The corresponding matric potentials in the sandy soil based on the best-fitted curve were -64 cm and -36 cm in the bulk soil, respectively. In the rhizosphere, the water content was elevated and reached 14% and 23% at these respective matric potentials. In loam, we observed a similar effect, where the matric potentials were -3492 cm and -385 cm at a water content of 10% and 20% in the bulk soil. Water content in the rhizosphere increased to 13% and 23%, respectively.
Estimated retention curves for bulk soil, rhizosphere, and the soil at the root-soil interface are shown for (a) sand and (b) loam. The rhizosphere represents the soil region between the soil surface and the soil region identified as rhizosphere extension. The soil at the root-soil interface represents the soil region approximately 0.315 mm from the root surface. The graph illustrates the calculated average soil water content in the rhizosphere and at the root-soil interface, plotted against the corresponding matric potential of the bulk soil. This assumes an equilibrium matric potential between the bulk soil and rhizosphere soil. Symbols represent the estimated retention curve data, while lines depict the best-fitted curves based on the bimodal Mualem-van Genuchten model
Discussion
We found that the rhizosphere of maize lateral roots is 1.26 times and of crown roots is 1.31 times wetter than the bulk soil. Our results showed that soil texture significantly impacted the water content in the vicinity of both lateral and crown roots, i.e., \({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\) were 16% higher in sandy soil compared to loamy soil (Fig. 3). In contrast, the soil moisture treatments had no significant impact on \({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\) of the crown root, whereas their effect on \({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\) of the lateral roots was significant.
The water content distribution (\({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\), and \({d}_{rh}\)) in the rhizosphere of newly emerging lateral and crown roots was significantly affected by soil texture, with sandy soil presenting higher \({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\) compared with loam (Fig. 3a and c). This divergence is coupled with an augmented rhizosphere extension in sandy soil (Fig. 5a and b), indicating a more efficient modulation of rhizosphere properties compared with loam. The combination of higher water content and extended rhizosphere in sandy soil suggests that roots in sandy soil may have exuded more mucilage than those in loamy soil, potentially accompanied by modifications in mucilage quality. Our reasoning is grounded in the findings of Kroener et al. (2018), who established a direct relationship between mucilage quantity (expressed in grams of dry mucilage per gram of dry soil) and its impact on water retention. Kroener et al. (2018) highlighted two critical points: 1) a minimum mucilage content is required to influence water retention in soils, and 2) when this critical mucilage content is reached, the effect on water holding capacity is more pronounced in fine-textured soil than in coarse-textured soil. Taking into account this information, we conclude that roots in sandy soil must have exuded more mucilage and/or mucilage with a greater impact on the water-holding capacity of the soil compared with loamy soil. Our explanation agrees with the findings of Santangeli et al. (2024), who measured the root exudation rate as µg C per unit of root surface area and found a higher exudation rate in sandy soil than in loamy soil during the developmental stage corresponding to BBCH 14, which aligns with the age of our plants. Notably, they used a maize variety and sand and loam similar to those used in this study, and their assessment was performed on maize plants grown in field conditions. Nazari et al. (2023) investigated mucilage collected from aerial nodal roots of maize varieties in different soil textures. They observed higher mucilage exudation in maize plants grown in loamy soil than in those grown in sandy clay loam, attributing this difference to increased microbial activity in loamy soil. Although the findings of Nazari et al. (2023) may initially appear contradictory to ours, a direct comparison is not justified, given that their study focused on fine-textured soils, unlike our examination of two contrasting textures.
Benard et al. (2021) presented a nuanced perspective on the processes governing the water-holding capacity of rhizosphere soil. Their investigation reveals that the mucilage content not only significantly influences this capacity but also underscores the pivotal role played by the quality of mucilage, as characterized by surface tension and viscosity. The study specifically highlights that mucilage with a higher viscosity leads to increased water retention in the soil. Viscosity, a critical attribute of mucilage, is directly correlated with its concentration in the liquid phase (Naveed et al. 2019; Benard et al. 2021). Moreover, the viscosity of mucilage is identified as another influential factor in determining the spatial distribution of mucilage around the roots and its concentration in the liquid phase. Plants may actively regulate the chemical and polymeric composition of mucilage to achieve a given mucilage viscosity and concentration in their vicinity. This aspect remains unexplored. Furthermore, the soil water content and chemical composition substantially affect the viscosity of mucilage within the soil matrix. Assuming a given exudation rate per unit root length, it is postulated that soils with a lower water content would exhibit a higher mucilage content within the liquid phase, resulting in an elevated viscosity (Naveed et al. 2019; Benard et al. 2021). Consequently, mucilage with higher viscosity tends to exhibit lower mobility within the soil, leading to an increased concentration at the root surface, whereas less viscous mucilage disperses farther away from the root surface. In addition to viscosity, the mobility and spatial distribution of mucilage within the soil influence its physical characteristics and diffusion (Holz et al. 2018). Sandy soils, characterized by higher diffusion coefficients at any given water content, exhibit enhanced mobility until they reach a critical water content where liquid continuity is no longer maintained (Holz et al. 2018; Moldrup et al. 2001). In addition, a lower interaction between the root exudation network and soil particles is anticipated in coarse-textured soils because of their smaller surface area and less actively charged surfaces, which results in higher mucilage mobility.
The higher \({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\), coupled with a greater \({d}_{rh}\) of newly emerged roots in sand compared with loam, could be attributed to mechanical and hydraulic protection of growing roots and/or strategic modification of the hydraulic contact with surrounding soils to facilitate water and nutrient uptake in coarse-textured soil. In our experimental setup, roots grown in sandy soil were subjected to less negative soil matric potential than those grown in loam, suggesting that a wetter rhizosphere in sandy soil cannot be attributed to only its potential impact on the hydraulic protection of roots from dehydration to soil. In general, coarse-textured soil is more prone to deteriorated contact between roots and soil as the soil dries. Helliwell et al. (2019) reported a higher soil porosity around the roots of plants grown in coarse-textured soil than in fine-textured soil, suggesting a more restricted contact between the root surface and liquid phase when the soil dries. A higher porosity around roots, particularly under soil drying conditions, results in lower contact between the root surface and the liquid phase. Plants may modify the mucilage exudation rate or its quality to make it more effective in the retention of water in coarse-textured soil and facilitate water and nutrient exchange under soil drying conditions. In addition, mucilage exudation decreases the penetration resistance of the roots into the soil. Maize plants increase the rhizodeposition of mucilage and root cap border cells in wet, compacted soil (Somasundaram et al. 2009). Because our sand had a higher bulk density, a higher mucilage exudation might reduce the friction around the growing roots. However, the measured effect of chia seed mucilage on the penetration resistance of the same soil textures used in our study showed that in loam with low water content, the resistance decreased but increased at high water contents (Rosskopf et al. 2022). The effect of mucilage addition on penetration resistance in sand was minor.
The moisture treatment had no effect on the \({\theta }_{rh}^{*}\) and \({d}_{rh}\) of crown roots grown in both sandy and loamy soils. In contrast, \({\theta }_{rh}^{*}\) and \({d}_{rh}\) of the lateral roots were significantly higher in dry conditions compared to wet conditions in loamy soil. This pattern was the opposite for lateral roots grown in sandy soil, where \({\theta }_{rh}^{*}\) and \({d}_{rh}\) were significantly higher in wet conditions compared to dry conditions. Given that elevated water content in the rhizosphere can be linked to the active modulation of rhizosphere properties by mucilage exudation, it is perplexing why \({\theta }_{rh}^{*}\) and \({d}_{rh}\) of crown roots were not affected by soil moisture treatment. In our experimental setup, the soil water potential among wet and dry treatments of sandy soils as well as in wet treatment of loamy soil was higher than -400 cm. Such a high soil matric potential does not yet induce any significant risk of root dehydration to the surrounding soils. Considering this point, we might not expect any significant difference in \({\theta }_{rh}^{*}\) and \({d}_{rh}\) between lateral and crown roots. However, if we consider the effect of mucilage on the facilitation of water and nutrient transport towards the root under drought conditions, we expect a significantly wetter rhizosphere around the root types active in water and nutrient uptake under drought conditions, particularly as we observed a reduced plant transpiration rate under dry conditions. On the other hand this risk starts to be relevant when we move to the dry loamy soil where the soil matric potential corresponding to our selected water content of 10% is around -3492 cm. This matric potential is estimated based on the matric potential of bulk soil (estimated from average water content), and the actual matric potential at the root-soil interface, particularly at the peak of transpiration, might be even more negative. In such conditions (dry loamy soil), lateral roots are more exposed to soil drying than crown roots, and plants might develop a wetter rhizosphere through mucilage exudation to protect newly emerged lateral roots. In contrast, crown roots exhibit notable distinctions from lateral roots, characterized by increased thickness and the presence of a protective multilayer of cells. Additionally, in response to drought, crown roots show increased lignification as protection against dehydration and exposure to very negative suction from soil, whereas lateral roots are more prone to drought (Hazman and Kabil 2022). Several studies have investigated the alteration of carbon partitioning in plants growing under drought stress conditions (Hasibeder et al. 2015; Palta and Gregory 1997; Hummel et al. 2010). Similarly, the beneficial characteristics of mucilage exudation under drought have been frequently discussed (Carminati et al. 2016; Ahmed et al. 2014; Somasundaram et al. 2009). However, we found no implication of a higher exudation rate due to dry conditions, except for lateral roots in loamy soil. This suggests that lateral roots in loamy soil may have a unique response to drought stress, possibly due to specific root architecture or localized soil conditions that enhance mucilage production and rhizosphere maintenance. Further investigation is needed to elucidate the underlying mechanisms governing these differential responses between root types and soil textures.
This study presents an in-situ estimation of the soil retention curve for a naturally formed rhizosphere, distinguishing it from previous retention curves derived from soils mixed with mucilage extracted from seeds or seedlings (Benard et al. 2019; Kroener et al. 2018; Naveed et al. 2019). Unlike prior approaches, our methodology does not involve knowledge of mucilage content, and mucilage exudation conditions differ due to the inherent heterogeneity of the soil. Nevertheless, we operate on the assumption that the bulk soil and rhizosphere reach equilibrium, particularly as our measurements were conducted during nighttime. If this assumption proves invalid, there is a risk of underestimating the retention curve of the rhizosphere, with potentially significant implications for the rhizosphere of roots that are more actively engaged in water uptake. As soil moisture decreases, the rate of root water uptake contributes to a drop in soil water potential around the roots, challenging the assumption of uniform water potential. Worth noting is that while direct comparison with existing data is not feasible, the shape and dynamics of our retention curve closely resemble those reported for extracted mucilage from chia seeds or seedlings when mixed with soils. Indeed, the difference between the water holding capacity of bulk soil and the rhizosphere is more pronounced in wet conditions, particularly in fine-textured soils than in coarse soils (Kroener et al. 2018; Benard et al. 2019; Naveed et al. 2019).
In conclusion, we present the effect of contrasting growth conditions (soil texture and soil moisture) on the development of a rhizosphere around the emerging crown and lateral roots of maize plants. We used the rhizosphere properties (\({\theta }_{rs}^{*}, {\theta }_{rh}^{*}\), \({d}_{rh}\)) as an indirect proxy for the modulation of rhizosphere hydraulic properties, where mucilage quantity and quality were considered the main drivers of changes. Generally, a wetter rhizosphere than bulk soil was observed in all treatments (\({\theta }_{rs}^{*}\) and \({\theta }_{rh}^{*}\)>1), however, soil moisture treatment had no significant impact on the observed water content profiles. As we observed a similar water content at the root-soil interface of both wet and dry treatments, we conclude that maize plants modulate their rhizosphere properties under dry conditions to ensure a critical minimum hydraulic contact with soil, identical to the one under wet conditions. In contrast, soil texture significantly affected the rhizosphere hydraulic properties, indicating stronger impacts of mucilage exudation in coarse-textured soil compared to fine-textured soil.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- \({\theta }_{rs}\) :
-
Vol. water content at the root-soil interface
- \({\theta }_{rh}\) :
-
Vol. water content in the rhizosphere
- \({\theta }_{rs}^{*}\) :
-
Normalized water content at the root-soil interface
- \({\theta }_{rh}^{*}\) :
-
Normalized water content in the rhizosphere
- \({d}_{rh}\) :
-
Rhizosphere extension from the root surface
References
Ahmed MA, Kroener E, Holz M, Zarebanadkouki M, Carminati A (2014) Mucilage exudation facilitates root water uptake in dry soils. Funct Plant Biol 41(11):1129. https://doi.org/10.1071/FP13330
Benard P, Zarebanadkouki M, Brax M, Kaltenbach R, Jerjen I, Marone F, Couradeau E, Felde VJMNL, Kaestner A, Carminati A (2019) Microhydrological niches in soils: how mucilage and EPS alter the biophysical properties of the rhizosphere and other biological hotspots. Vadose Zone J 18(1):180211. https://doi.org/10.2136/vzj2018.12.0211
Benard P, Schepers JR, Crosta M, Zarebanadkouki M, Carminati A (2021) Physics of viscous bridges in soil biological hotspots. Water Resour Res 57(11):e2021WR030052. https://doi.org/10.1029/2021WR030052
Carminati A, Javaux M (2020) Soil rather than xylem vulnerability controls stomatal response to drought. Trends Plant Sci 25(9):868–880. https://doi.org/10.1016/j.tplants.2020.04.003
Carminati A, Moradi AB, Vetterlein D, Vontobel P, Lehmann E, Weller U, Vogel H-J, Oswald SE (2010) Dynamics of soil water content in the rhizosphere. Plant Soil 332(1):163–176. https://doi.org/10.1007/s11104-010-0283-8
Carminati A, Zarebanadkouki M, Kroener E, Ahmed MA, Holz M (2016) Biophysical rhizosphere processes affecting root water uptake. Ann Bot 118(4):561–571. https://doi.org/10.1093/aob/mcw113
Carminati A, Benard P, Ahmed MA, Zarebanadkouki M (2017) Liquid bridges at the root-soil interface. Plant Soil 417(1):1–15. https://doi.org/10.1007/s11104-017-3227-8
Comas L, Becker S, Cruz VM, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4. https://www.frontiersin.org/article/https://doi.org/10.3389/fpls.2013.00442
Draye X, Kim Y, Lobet G, Javaux M (2010) Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. J Exp Bot 61(8):2145–2155. https://doi.org/10.1093/jxb/erq077
Gardner WR (1991) Modeling water uptake by roots. Irrig Sci 12(3):109–114. https://doi.org/10.1007/BF00192281
Hasibeder R, Fuchslueger L, Richter A, Bahn M (2015) Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol 205(3):1117–1127. https://doi.org/10.1111/nph.13146
Hazman MY, Kabil FF (2022) Maize root responses to drought stress depend on root class and axial position. J Plant Res 135(1):105–120. https://doi.org/10.1007/s10265-021-01348-7
Helliwell JR, Sturrock CJ, Miller AJ, Richard Whalley W, Mooney SJ (2019) The role of plant species and soil condition in the structural development of the rhizosphere. Plant Cell Environ 42(6):1974–1986. https://doi.org/10.1111/pce.13529
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005) Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol 168(2):293–303. https://doi.org/10.1111/j.1469-8137.2005.01512.x
Holz M, Zarebanadkouki M, Kaestner A, Kuzyakov Y, Carminati A (2018) Rhizodeposition under drought is controlled by root growth rate and rhizosphere water content. Plant Soil 423(1–2):429–442. https://doi.org/10.1007/s11104-017-3522-4
Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, Christophe A et al (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol 154(1):357–372. https://doi.org/10.1104/pp.110.157008
Iijima M, Higuchi T, Barlow PW (2004) Contribution of root cap mucilage and presence of an intact root cap in maize (Zea Mays) to the reduction of soil mechanical impedance. Ann Bot 94(3):473–477. https://doi.org/10.1093/aob/mch166
Kroener E, Zarebanadkouki M, Kaestner A, Carminati A (2014) Nonequilibrium water dynamics in the rhizosphere: how mucilage affects water flow in soils. Water Resour Res 50(8):6479–6495. https://doi.org/10.1002/2013WR014756
Kroener E, Holz M, Zarebanadkouki M, Ahmed M, Carminati A (2018) Effects of mucilage on rhizosphere hydraulic functions depend on soil particle size. Vadose Zone J 17(1):170056. https://doi.org/10.2136/vzj2017.03.0056
Liebersbach H, Steingrobe B, Claassen N (2004) Roots regulate ion transport in the rhizosphere to counteract reduced mobility in dry soil. Plant Soil 260(1):79–88. https://doi.org/10.1023/B:PLSO.0000030191.92338.6a
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112(2):347–357. https://doi.org/10.1093/aob/mcs293
McCully ME, Boyer JS (1997) The expansion of maize Root-Cap mucilage during hydration. 3. changes in water potential and water content. Physiol Plant 99(1):169–177. https://doi.org/10.1111/j.1399-3054.1997.tb03445.x
Moldrup P, Olesen T, Komatsu T, Schjønning P, Rolston DE (2001) Tortuosity, diffusivity, and permeability in the soil liquid and gaseous phases. Soil Sci Soc Am J 65(3):613–623. https://doi.org/10.2136/sssaj2001.653613x
Moradi AB, Carminati A, Vetterlein D, Vontobel P, Lehmann E, Weller U, Hopmans JW, Vogel H-J, Oswald SE (2011) Three-dimensional visualization and quantification of water content in the rhizosphere. New Phytol 192(3):653–663. https://doi.org/10.1111/j.1469-8137.2011.03826.x
Naveed M, Ahmed MA, Benard P, Brown LK, George TS, Bengough AG, Roose T, Koebernick N, Hallett PD (2019) Surface tension, rheology and hydrophobicity of rhizodeposits and seed mucilage influence soil water retention and hysteresis. Plant Soil 437(1):65–81. https://doi.org/10.1007/s11104-019-03939-9
Nazari M, Bilyera N, Banfield CC, Mason-Jones K, Zarebanadkouki M, Munene R, Dippold MA (2023) Soil, climate, and variety impact on quantity and quality of maize root mucilage exudation. Plant Soil 482(1):25–38. https://doi.org/10.1007/s11104-022-05669-x
Oburger E, Jones DL (2018) Sampling root exudates – mission impossible? Rhizosphere 6(June):116–133. https://doi.org/10.1016/j.rhisph.2018.06.004
Palta JA, Gregory PJ (1997) Drought affects the fluxes of carbon to roots and soil in 13C pulse-labelled plants of wheat. Soil Biol Biochem 29(9):1395–1403. https://doi.org/10.1016/S0038-0717(97)00050-3
Perktold J, Seabold S, Sheppard K, Fulton C, Shedden K, jbrockmendel, j-grana6, et al (2024) “Statsmodels/Statsmodels: Release 0.14.2.” Zenodo. 10.5281/zenodo.10984387
Read DB, Bengough AG, Gregory PJ, Crawford JW, Robinson D, Scrimgeour CM, Young IM, Zhang K, Zhang X (2003) Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol 157(2):315–326. https://doi.org/10.1046/j.1469-8137.2003.00665.x
Rosskopf U, Uteau D, Peth S (2022) Effects of mucilage concentration at different water contents on mechanical stability and elasticity in a loamy and a sandy soil. Eur J Soil Sci 73(1):e13189. https://doi.org/10.1111/ejss.13189
Santangeli M, Steininger-Mairinger T, Vetterlein D, Hann S, Oburger E (2024) Maize (Zea Mays L.) root exudation profiles change in quality and quantity during plant development – a field study. Plant Sci 338(January):111896. https://doi.org/10.1016/j.plantsci.2023.111896
Somasundaram S, Rao TP, Tatsumi J, Iijima M (2009) Rhizodeposition of mucilage, root border cells, carbon and water under combined soil physical stresses in Zea Mays L. Plant Prod Sci 12(4):443–448. https://doi.org/10.1626/pps.12.443
Vetterlein D, Lippold E, Schreiter S, Phalempin M, Fahrenkampf T, Hochholdinger F, Marcon C et al (2021) Experimental platforms for the investigation of spatiotemporal patterns in the rhizosphere—laboratory and field scale. J Plant Nutr Soil Sci 184(1):35–50. https://doi.org/10.1002/jpln.202000079
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in python. Nat Methods 17(3):261–272. https://doi.org/10.1038/s41592-019-0686-2
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132(1):44–51
Zarebanadkouki M, Ahmed MA, Carminati A (2016) Hydraulic conductivity of the root-soil interface of lupin in sandy soil after drying and rewetting. Plant Soil 398(1):267–280. https://doi.org/10.1007/s11104-015-2668-1
Zarebanadkouki M, Fink T, Benard P, Banfield CC (2019) Mucilage facilitates nutrient diffusion in the drying rhizosphere. Vadose Zone J 18(1):190021. https://doi.org/10.2136/vzj2019.02.0021
Zhan Ai, Schneider H, Lynch JP (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168(4):1603–1615. https://doi.org/10.1104/pp.15.00187
Acknowledgements
Seeds of the maize wild type B73 were provided by Caroline Macron and Frank Hochholdinger (University Bonn). The aluminum containers were kindly provided by Sascha Oswald (University Potsdam).
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was carried out in the framework of the priority programme 2089. “Rhizosphere spatiotemporal organisation—a key to rhizosphere functions” funded by DFG, German Research Foundation (project number 470748155).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ruth Adamczewski and Mohsen Zarebanadkouki. The first draft of the manuscript was written by Ruth Adamczewski and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Hans Lambers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adamczewski, R., Kaestner, A. & Zarebanadkouki, M. Rhizosphere hydraulic regulation in maize: tailoring rhizosphere properties to varying soil textures and moistures. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06840-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06840-2